Summary
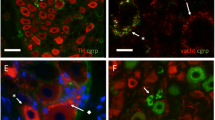
To determine the reaction of adrenergic ganglion cells and small intensely fluorescent (SIF) cells to chronic deafferentation, catecholamine fluorescence of the major pelvic ganglion (MPG) of the rat has been studied following section of the hypogastric nerve, pelvic nerve and sympathetic trunk. Only minor changes occurred following section of the hypogastric nerve; the fluorescence surrounding a few adrenergic ganglion cells became brighter. In contrast, pelvic neurectomy resulted in the appearance of numerous varicose fibres and an increase in the fluorescent intensity of fibres enclosing many ganglion cells. Varicose fibres seem to originate from adrenergic ganglion cells and SIF cells. In many instances, nests of SIF cells gave rise to radially oriented fibres. Removal of the sympathetic trunk appeared to have no effect on the MPG. It is suggested that the appearance of varicose fibres from SIF cells following deafferentation may be due to collateral sprouting of these cells or to the increased fluorescence of pre-existing processes.
Similar content being viewed by others
References
Axelsson, A., Björklund, A., Falck, B., Lindvall, O. andSvennson, L. A. (1973) Glyoxylic acid condensation: a new fluorescence method for the histochemical demonstration of biogenic amines.Acta physiologica scandinavica 87, 57–62.
Björklund, A., Lindvall, O. AndSvennson, L. A. (1972) Mechanisms of fluorophore formation in the histochemical glyoxylic acid method for monoamines.Histochemie 32, 113–31.
Björklund, A. andLindvall, O. (1975) Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals.Brain Research 83, 531–37.
Brudin, J. (1965) Distribution and function of adrenergic nerves in the rabbit Fallopian tube.Acta physiologica scandinavica 66, Suppl.259, 1–57.
Chiba, T. andWilliams, T. H. (1975) Histofluorescence characteristics and quantification of small intensely fluorescent (SIF) cells in sympathetic ganglia of several species.Cell and Tissue Research 162, 331–41.
Costa, M. andEränkö, O. (1974). Histochemical correlates of cold-induced transsynaptic induction in the rat superior cervical ganglion.Histochemical Journal 6, 35–53.
Dahlström, A. andFuxe, K. (1964) A method for the demonstration of adrenergic nerve fibres in peripheral nerves.Zeitschrift für Zellforschung und mikroskopische Anatomie 62, 602–7.
Dail, W. G. (1976a) Adrenergic fluorescence in the major pelvic ganglion of the rat after denervation.Anatomical Record 184, 387 (Abstracts).
Dail, W. (1976b) Histochemical and fine structural studies of SIF cells in the major pelvic ganglion of the rat. In:SIF Cells, Structure and Function of the Small Intensely Fluorescent Sympathetic Cells (edited byEränkö, O.), pp. 8–18. Fogarty International Center Proceedings No. 30, DHEW Publication No. (NIH) 76-942.
Dail, W. G. andEvan, A. P. (1974) Experimental evidence indicating that the penis of the rat is innervated by short adrenergic neurons.American Journal of Anatomy 141, 203–18.
Dail, W. G., Evan, A. P. andEason, H. R. (1975) The major ganglion in the pelvic plexus of the male rat. A histochemical and ultrastructural study.Cell and Tissue Research 159, 49–62.
de La Torre, J. C. andSurgeon, J. W. (1976) A methodological approach to rapid and sensitive monoamine histofluorescence using a modified glyoxylic acid technique: The SPG method.Histochemistry 49, 81–93.
El Badawi, A. andSchenk, E. A. (1967) Histochemical methods for separate, consecutive and simultaneous demonstration of acetylcholine and norepinephrine in cryostat sections.Journal of Histochemistry and Cytochemistry 15, 580–8.
Elfvin, L-G. (1971) Ultrastructural studies on the synaptologv of the inferior mesenteric ganglion of the cat. I. Observations on the cell surface of the postganglionic perikarya.Journal of Ultrastructure Research 37, 411–25.
Elfvin, L. G., Hökfelt, T. andGoldstein, M. (1975) Fluorescence microscopical, immunohistochemical and ultrastructural studies on sympathetic ganglia of the guinea pig, with special reference to the SIF cells and their catecholamine content.Journal of Ultrastructure Research 51, 377–96.
ErÄnkö, O. andHärkönen, M. (1963) Histochemical demonstration of fluorogenic amines in the cytoplasm of sympathetic ganglion cells of the rat.Acta physiologica scandinavica 58, 285–6.
Eränkö, O. andHärkönen, M. (1965) Effect of axon division on the distribution of noradrenaline and acetylcholinesterase in sympathetic neurons of the rat.Acta physiologica scandinavica 63, 411–2.
Giacobini, E., Karjalainen, K., Kerpel-Fronius, S. andRitzen, M. (1970) Monoamines and monoamine oxidase in denervated sympathetic ganglia of the cat.Neuropharmacology 9, 59–66.
Hamberger, B., Norberg, K-A. andSjöqvist, F. (1963) Evidence for adrenergic nerve terminals and synapses in sympathetic ganglia.International Journal of Neuropharmacology 2, 279–82.
Hamberger, B., Norberg, K-A. andUngerstedt, U. (1965) Adrenergic synaptic terminals in autonomic ganglia.Acta physiologica scandinavica 64, 285–6.
Härkönen, M. (1964) Carboxylic esterases, oxidative enzymes and catecholamines in the superior cervical ganglion of the rat and the effect of pre- and post-ganglionic nerve division.Acta physiologica scandinavica 63, Suppl.237, 1–94.
Jacobowitz, D. (1970) Catecholamine fluorescence studies of adrenergic neurons and chromaffin cells in sympathetic ganglia.Federation Proceedings 29, 1929–44.
Jacobowitz, D. M. andGreene, L. A. (1974) Histofluorescence study of chromaffin cells in dissociated cell cultures of chick embryo sympathetic ganglia.Journal of Neurobiology 5, 65–83.
Jacobowitz, D. M. (1974) The Peripheral Autonomic Nervous System. In:The Peripheral Nervous System (edited byHubbard, J. I.). New York: Plenum Press.
Kanerva, L. (1971) The postnatal development of monoamines and cholinesterases in the paracervical ganglion of the rat uterus.Progress in Brain Research 34, 433–44.
Krinke, G., Schinder, K. andHess, R. (1974) Quantitation of noradrenaline fluorescence in the superior cervical ganglion of the rat and the effect of postganglionic axotomy.Experientia 30, 37–8.
Langworthy, O. R. (1965) Innervation of the pelvic visceral organs of the rat.Investigative Urology 2, 491–511.
Libet, B. andBowman, Ch. (1974). Concomitant changes in formaldehyde-induced fluorescence of dopamine interneurons and in slow inhibitory postsynaptic potentials of the rabbit superior cervical ganglion, induced by stimulation of the preganglionic nerve or by a muscarinic agent.Journal of Physiology 237, 635–62.
Matthews, M. A. (1971) Evidence from degeneration experiments for the preganglionic origin of afferent fibres to the small granule-containing cells of the rat superior ganglion.Journal of Physiology 218, 95.
Matthews, M. andRaisman, G. (1969). The ultrastructure and somatic efferent synapses of small granule-containing cells in the superior cervical ganglion.Journal of Anatomy 105, 255–82.
Norberg, K-A. andSjöqvist, F. (1966) New possibilities for adrenergicmodulation of ganglionic transmission.Pharmacological Reviews 18, 743–51.
Olson, L. (1970) Fluorescence histochemical evidence for axonal growth and secretion from transplanted adrenal medullary tissue.Histochemie 22, 1–7.
Olson, L. andMalmfors, T. (1970) Growth characteristics of adrenergic nerves in the adult rat.Acta physiologica scandinavica Suppl.348, 1–112.
Owman, Ch. andSjöberg, N. O. (1966) Adrenergic nerves in the female genital tract of the rabbit. With remarks on cholinesterase-containing structures.Zeitschrift für Zellforschung und mikroskopische Anatomie 74, 182–97.
Owman, Ch. andSjöberg, N. O. (1967) Difference in rates of depletion and recovery of noradrenaline in ‘short’ and ‘long’ sympathetic nerves after reserpine treatment.Life Sciences 6, 2549–56.
Raisman, G., Field, P. M., Ostberg, A. J. C. Iversen, L. I. andZigmond, R. F. (1974) A quantitative ultrastructural and biochemical analysis of the process of reinnervation of the superior cervical ganglion in the adult rat.Brain Research 71, 1–16.
Siegrist, G., Dolivo, M., Dunant, Y., Foroglu-Kerameus, C., De Ribaupierre, F. andRouiller, C. (1968) Ultrastructure and function of the chromaffin cells in the superior cervical ganglion of the rat.Journal of Ultrastructure Research 25, 381–407.
Sjöstrand, N. O. (1962) Effect of reserpine and hypogastric denervation on the noradrenaline content of the vas deferens and seminal vesicle of the guinea pig.Acta physiologica scandinavica 56, 376–80.
Sjöstrand, N. O. (1965) The adrenergic innervation of the vas deferens and the accessory male genital glands.Acta physiologica scandinavica 65, Suppl.257, 1–182.
Sladek, J. R. Jr. andParnavelas, J. G. (1975) Catecholamine-containing dendrites in primate brain.Brain Research 100, 657–62.
Taxi, J. andMikulajova, M. (1976) Some cytochemical and cytological features of the so-called SIF cell of the superior cervical ganglion of the rat.Journal of Neurocytology 5, 283–95.
Van Orden, L. S. III, Burke, J. P., Geyer, M. andLodoen, F. V. (1970) Localization of depletion-sensitive and depletion-resistant norepinephrine storage sites in autonomic ganglia.Journal of Pharmacology and Experimental Therapeutics 174, 56–71.
Von Euler, U. S. andLishajko, F. (1966) A specific kind of noradrenaline granule in the vesicular gland and vas deferens of the bull.Life Sciences 5, 687–91.
Williams, T. H. (1967) Electron microscopic evidence for an autonomic interneuron.Nature 214, 309–10.
Williams, T. H. andPalay, S. L. (1969) Ultrastructure of the small neurons in the superior cervical ganglion.Brain Research 15, 17–34.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dail, W.G., Evan, A.P. Effects of chronic deafferentation on adrenergic ganglion cells and small intensely fluorescent cells. J Neurocytol 7, 25–37 (1978). https://doi.org/10.1007/BF01213458
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01213458