Abstract
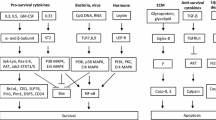
Apoptosis is a physiological process of cell death that occurs in all multicellular organisms. Its dysregulation has been postulated as one of the main causes in the development of diseases such as cancer, AIDS, autoimmune diseases and allergy. Apoptosis has been mainly studied in the inflammatory cells that participate in the late and chronic stages of allergy (eosinophils, neutrophils, lymphocytes and macrophages) as a new way to elucidate the pathogenesis of this disease. Nevertheless, much less it is known about the regulation of apoptosis in the “initiators” of the allergic process: The Mast Cells. In normal conditions, mast cells are described as long-living cells that keep a constant number of cells in tissues. However, increased numbers of mast cells are observed in the late phase of asthma and in both the inflammatory and in the repair/remodeling stage of various inflammatory/fibrotic disorders. In this report, we discuss the possible mechanisms that regulate the apoptotic process in normal conditions and disease, such as survival factors and death receptors. A link between mast cell activation, during the early stages of the allergic process, and triggering of anti-apoptotic signaling pathways is also suggested as an important contributor to the extended life of mast cells.
Similar content being viewed by others
References
Ekert PG, Vaux DL. Apoptosis and the immune system. Br Med Bull 1997; 53:591–603.
Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci USA 1996; 76: 777–779.
Dragovich T, Rudin CM, Thompson CB. Signal transduction pathways that regulate cell survival and cell death. Oncogene 1998; 17: 3207–3213.
Chao DT, Korsmeyer SJ. Bcl-2 family: Regulation of cell death. Ann Rev Imm 1998; 16: 395–419.
Oltvai ZN, Korsmeyer SJ. Checkpoints of dueling dimers oil death whishes. Cell 1994; 79: 189–192.
Yang E, Korsmeyer SJ. Molecular thanatopsis: A discourse on the BCL2 family and cell death. Blood 1996; 88: 386–401.
Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2 displaces Bax and promotes cell death. Cell 1995; 80: 285–291.
Vander-Heiden MG, Chandel NS, Williamson EK, Schumaker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 1997; 91: 627–637.
Evan G, Littlewood T. A matter of life and cell death. Science 1998; 281: 1317–1322.
Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev 1996; 10: 2401–2410.
Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induce phosphorylation of p53 alleviates inhibition by MDM2. Cell 1997; 91: 325–334.
Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: Activation, costimulation and death. Cell 1994; 76: 959–962.
Nagata S. Apoptosis by death factor. Cell 1997; 88: 355–365.
Brojatsch J, Naughton J, Rolls MM, Zingler K, Young JA. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 1996; 87: 845–855.
Chinnaiyan AM, O'Rourke K, Yu GG, Lyores RH, Garg M, Duan DR. Signal transduction by DR3, a death domaincontaining receptor related to TNFR1 and CD95. Science. 1996; 274: 990–992.
Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytolitic ligand TRAIL. Science 1997; 276: 111.
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonistic decoy receptor and a death domain-containing receptor for TRAIL. Science 1997; 277: 815.
Sheridan JP, Masters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, et al. Control of TRAIL-induced apoptosis by a family of Signaling and decoy receptors. Science 1997; 277: 818–821.
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N. TRAIL-R2:Anovel apoptosis-mediating receptor for TRAIL. EMBO J 1997; 16: 5386–5397.
Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ, Bell JI. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol 1997; 7: 693–696.
MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Almenri T, Cohen GM, Alnemri ES. Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem 1997; 272: 25417–25420.
Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J. Characterization of two receptors for TRAIL. FEBS Lett 1997; 416: 329.
Pan G, Bauer JH, Haridas V, et al. Identification and functional characterization of DR6, a novel death domain-containingTNF receptor. FEBS 1998; 431: 351–356.
Liepinsh E, Ilg LL, Otting G, Ibanez CF. NMR structure of the death domain of the p75 neurotrophin receptor.EMBO J 1997; 16: 4999–5005.
Baker SJ, Premkumar Reddy E. Modulation of life and death by the TNF receptor superfamily. Oncogene 1998; 17: 3261–3270.
Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 Kd TNF receptor signal cell death. Cell 1993; 74: 845–853.
Vaux DL, Haecker G, Strasser A. An evolutionary perspective on apoptosis. Cell 1994; 76: 777–779.
Wagner AJ, Kokontis JM, Hay N. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21wafl/cipl. Genes Dev 1994; 8: 2817–2830.
Plaut M, Pierce JH, Watson CJ, et al. Mast cell lines produce lymphokines in response to cross-linkeage of Fc epsilon RI or to calcium ionophores. Nature 1989; 339: 64–67.
Ohta K and Yamashita N. Apoptosis of eosinophils and lymphocytes in allergic inflammation. J Allergy Clin Immunol 1999; 104: 14–21.
Simon H-U, Yousefi S, Schranz C, Shapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol 1997; 158: 3902–3908.
Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990; 323: 1033–1039.
Jayaraman S, Castro M, O'Sullivan M, Bragdon MJ, Holtzman MJ. Resistance to Fas-mediated T cell apoptosis in asthma. J Immunol 1999; 162: 1717–1722.
Vignola AM, Chanez P, Chiappara G, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol 1999; 103: 563–573.
Simon H-U, Blaser K. Inhibition of programmed eosinophil death: A key pathogenic event for eosinophilia? Immunol Today 1995; 16: 53–55.
KitamuraY, Shimada M, Hatanaka K, MiyanoY. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature 1977; 268: 442–443.
Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev 1997; 77: 1033–1079.
Stevens RL, Fox CC, Lichtenstein LM, Austen KF. Identification of chondroitin sulfate E proteoglycans and heparin proteoglycans in the secretory granules of human lung mast cells. Proc Natl Acad Sci USA 1988; 85: 2284–2287.
Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol Today 1990; 11: 458–464.
Schwartz L, Huff T. Biology of mast cells and basophils. In: Middlleton E Jr., Reed CE, Ellis EF, Adkinson FN, Yunginger JW, Busse WW, eds. Allergy. Principles and Practice. Missouri: Mosby 1993: 135–168.
Coffman RL. T-helper heterogeneity and immune response patterns. Hosp Pract 1989; 24: 101–105.
Church MK, Levi-Schaffer F. The human mast cell. J Allergy Clin Immunol 1997; 2: 155–160.
Bradding P, Okayama Y, Howarth PH, Church MK, Holgate ST. Heterogeneity of human mast cells based on cytokine content. J Immunol 1995; 155: 297–307.
Crimi E, Chiaramondia M, Milanese M, Rossi GA, Brusasco V. Increased numbers of mast cells in bronchial mucosa after the late phase asthmatic response to allergen. Am Rev Respir Dis 1991; 144: 1282–1286.
Levi-Schaffer F, Segal V, Mekori YA, Claman HN, Hammel I. Morphological evidence for chronic mast cell activation after prolonged exposure with supernatants from chronic graft-versus-host splenocytes. Immunol Letters 1991; 27: 13–18.
Nishioka K, Kobayashi Y, Katayama I, Takijiri C. Mast cell numbers in diffuse scleroderma. Arch Dermatol 1987; 123: 205–208.
Armbrust T, Batusic D, Ringe B, Ramadori G. Mast cells distribution in human liver disease and experimental rat liver fibrosis. Indications for mast cell participation in development of liver fibrosis. J Hepatol 1997; 26: 1042–1054.
Gelbmann CM, Mestermann S, Gross V, Kollinger M, Scholmerich J, Falk W. Strictures in Crohn's disease are characterized by an accumulation of mast cells colocalised with laminin but not with fibronectin or vitronectin. Gut 1999; 45: 210–217.
Galli SJ. Mast Cells and basophils. Curr Opin Hematol 2000; 7: 32–39.
Metcalfe DD, Mekori JA, Rottem M. Mast cell ontogeny and apoptosis. Exp Dermatol 1995; 4: 227–230.
Mekori YA, Oh CK, Metcalfe DD. IL-3-dependent murine mast cells undergo apoptosis on removal of IL-3. Prevention of apoptosis by c-kit ligand. J Immunol 1993; 151: 3775–3784.
Dvorak AM, Seder RA, Paul WE, Morgan ES, Galli SJ. Effects of interleukin-3 with or without the c-kit ligand, stem cell factor, on the survival, and cytoplasmic granule formation of mouse basophils and mast cells in vitro. Am J Pathol 1994; 144: 160–170.
Iemura A, Tsai M, Akikazu A, Wershil BK, Galli SK. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol 1994; 144: 321–328.
Yee NS, Paek I, Besner P. Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: Basis for radiosensitivity of white spotting and steel mutant mice. J Exp Med 1994; 179: 1777–1787.
Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: An essential role for Rac1 and JNK activation in mast cell proliferation.EMBO J 1998; 17: 6250–6262.
Gommerman JL, Berger SA. Protection from apoptosis by steel factor but not interleukin-3 is reversed through blockade of calcium influx. Blood 1998; 91: 1891–1900.
Ito F, Toyota N, Sakai H, Takahashi H, Iizuka H. FK506 and cyclosporin A inhibit stem cell factor-dependent cell proliferation/ survival, while inducing upregulation of c-kit expression in cells of the mast cell line MC/9. Arch Dermatol Res 1999; 291: 275–283.
Mekori YA, Metcalfe DD. Transforming growth factor-beta prevents stem cell factor-mediated rescue of mast cells from apoptosis after IL-3 deprivation. J Immunol 1994; 153: 2194–2203.
Oskeritzian CA, Wang Z, Kochan JP, Grimes M, Du Z, Chang H-W. Recombinant human (rh) IL-4-mediated apoptosis and recombinant human IL-6-mediated protection of recombinant human stem cell factor-dependent human mast cells derived from cord blood mononuclear cell progenitors. J Immunol 1999; 163: 5105–5115.
Bischoff SC, Sellge G, Lorentz A, Sebald W, Raab R, Manns MP. IL-4 enhances proliferation and mediator release in mature human mast cells. Proc Natl Acad Sci USA 1999; 96: 8080–8085.
Yanagida M, Fukamachi H, Takei M, et al. Interferon-gamma promotes the survival and Fc epsilon RI-mediated histamine release in cultured human mast cells. Immunology 1996; 89: 547–552.
Demoulin JB, Uyttenhove C, Van Roost E, et al. A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol Cell Biol 1996; 16: 4710–4716.
Kawamoto K, Okada T, Kannan Y, Ushio H, Matsumoto M, Matsuda H. Nerve growth factor prevents apoptosis of rat peritoneal mast cells through the trk proto-oncogene receptor. Blood 1995; 86: 4638–4644.
Aloe L. The effect of nerve growth factor and its antibody on mast cells in vivo. J Neuroimmunol 1988; 18: 1–12.
Bullock ED, Johnson Jr EM. Nerve growth factor induces the expression of certain cytokine genes and bcl-2 in mast cells. J Biol Chem 1996; 271: 27500–27508.
Yoon SO, Casaccia-Bonnefil P, Carter B, ChaoMV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neuroscience 1998; 18: 3273–3281.
Jippo T, Ushio H, Hirota S, et al. Poor response of cultured mast cells derived from mi/mi mutant mice to nerve growth factor. Blood 1994; 84: 2977–2983.
Tam SY, Tsai M, Yamaguchi M, Yano K, Butterfield JH, Galli SJ. Expression of functional TrkA receptor tyrosine kinase in the HMC-1 human mast cell line and in human mast cells. Blood. 1997; 90: 1807–1820.
Cervero C, Escribano L, San Miguel JF, et al. Expression of Bcl-2 by human bone marrow mast cells and its overexpression in mast cell leukemia. Am J Hematol 1999; 60: 191–195.
Hartmann K, Zirbes TK, Hasan S, et al. Expression of the antiapoptotic protein Bcl-X in human mast cells and mastocytosis. J Allergy Clin Immunol 2000; 105: S66 (abstract).
Hartmann K, Wagelie-Steffen AL, von Stebut E, Metcalfe DD. Fas (CD95, APO-1) antigen expression and function in murine mast cells. J Immunol 1997; 159: 4006–4014.
Wagelie-Steffen AL, Hartmann K, Vliagoftis H, Metcalfe DD. Fas ligand (FasL, CD95L, APO-1L) expression in murine mast cells. Immunology 1998; 94: 569–574.
Nilsson G, Blom T, Kusche-GullbergM, et al.Phenotypic characterization of the human mast cell line HMC-1. Scand J Immunol 1994; 39: 489–498.
Piliponsky AM, Daigle I, Simon H-U, Levi-Schaffer F. Expression and functionality of death receptors on the human mast cell line HMC-1. J Allergy Clin Immunol 2000; 105: S63 (abstract).
Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T Cell Apoptosis. Immunity 1998; 8: 615–623.
Shalit M, Levi-Schaffer F. Challenge of mast cells with increasing amounts of antigen induces desensitization. Clin Exp Allergy 1995; 25: 896–902.
Yoshikawa H, Nakajima Y, Tasaka K. Glucocorticoid suppresses autocrine survival of mast cells by inhibiting IL-4 production and ICAM-1 expression. J Immunol 1999; 162: 6162–6170.
Ishizuka T, Chayama K, Takeda K, et al. Mitogen-Activated Protein Kinase activation through Fcɛ receptor I and stem cell factor receptor is differentially regulated by phosphatidylinositol 3-kinase and calcineurin in mouse bone marrow-derived mast cells. J Immunol 1999; 162: 2087–2094.
Wang H-G, Pathan N, Ethell IM, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999; 284: 339–343.
Liu Q, SasakiT, Kozieradzki I, et al. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev 1999; 13: 786–791.
Damen JE, Liu L, Cutler RL, Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-kd tyrosine phosphorylated protein. Blood 1993; 82: 2296–2303.
Hemmings BA. Signal transduction-Akt signaling: Linking membrane events to life and death decision. Science 1997; 275: 628–630.
Toth R, Szegesdi E, Molnar G, Lord JM, Fesus L, Szondy Z. Regulation of cell surface expression of Fas (CD95) ligand and susceptibility to Fas (CD95)-mediated apoptosis in activationinduced T cell death involves calcineurin and protein kinase C, respectively. Eur J Immunol 1999; 29: 383–393.
Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 1992; 357: 695–697.
Scheid MP, Lauener RW, Duronio V. Role of phosphotidylinositol 3-OH-kinase activity in the inhibition of apoptosis in haemopoietic cells: Phosphatidylinositol 3-OH-kinase inhibitors reveal a difference in signaling between interleukin-3 and granulocyte-macrophage colony stimulating factor. J Biochem 1995; 312: 159–162.
Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol 1996; 80: S40-S45.
Tang B, Mano H, Yi HT, Ihle JN. Tec kinase associates with c-kit and is tyrosine phosphorylated and activated following stem cell factor binding. Mol Cell Biol 1994; 14: 8432–8437.
Kawakami Y, Yao L, Tsukada S, Witte ON, Kawakami T. Tyrosine phosphorylation and activation of Bruton tyrosine kinase upon FcɛRI cross-linking. Mol Cell Biol 1994; 14: 5108–5113.
Kawakami Y, Miura T, Bissonnette R, et al. Bruton's tyrosine kinase regulates apoptosis and JNK/SAPK kinase activity. Proc Natl Acad Sci USA 1997; 94: 3938–3942.
Kawakami Y, Hartman SE, Holland PM, Cooper JA, Kawakami T. Multiple signalling pathways for the activation of JNK in mast cells: Involvement of Bruton's Tyrosine Kinase, Protein Kinase C, and JNK Kinases, SEK1 and MKK7. J Immunol 1998; 161: 1795–1802.
de Paulis A, Minopoli G, Arbustini E, et al. Stem Cell Factor is localized in, released from, and cleaved by human mast cells. J Immunol 1999; 163: 2799–2808.
Lavker RM, Schechter NM. Cutaneous mast cell depletion results from topical corticosteroids usage. J Immunol 1985; 135: 2368–2373.
Scheleimer RP. The effects of glucocorticoids on mast cells and basophils. In: Schleimer RP, Claman HN, Oronski A, eds. Anti-Inflammatory steroids action: Basic and clinical aspects. San Diego: Academic Press 1989: 226–258.
King, SJ. Miller HRP, Newlands GFJ, Woodbury RG. Depletion of mucosal mast cell protease by corticosteroids: Effect on intestinal anaphylaxis in the rat. Proc Natl Acad Sci USA 1986; 82: 1214–1218.
Finotto S, Mekori YA, Metcalfe DD. Glucocorticoids decrease tissue mast cell number by reducing the production of the c-kit ligand, stem cell factor, by resident cells. J Clin Invest 1997; 99: 1721–1728.
Kassel O, Schmidlin F, Duvernelle C, de Blay F, Frossard N. Upand Down-Regulation by glucocorticoids of the constitutive expression of the mast cell growth factor stem cell factor by human lung fibroblasts in culture. Mol Pharmacol 1998; 54: 1073–1079.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piliponsky, A.M., Levi-Schaffer, F. Regulation of apoptosis in mast cells. Apoptosis 5, 435–441 (2000). https://doi.org/10.1023/A:1009680500988
Issue Date:
DOI: https://doi.org/10.1023/A:1009680500988