Summary
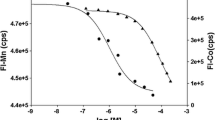
The clonedfur (ferric uptake regulation) gene ofEscherichia coli K12 was ligated to an expression vector which was inducible with nalidixic acid. The Fur protein was isolated in a single step by immobilized metal-ion-affinity chromatography over zinc iminodiacetate agarose. The amino acid composition of the isolated protein agreed with that predicted from the gene sequence and indicated post-transcriptional removal of the N-terminal methionine residue. All four cysteines were shown to be present as thiols. Proteolysis with trypsin and chymotrypsin yielded large fragments identifiable on polyacrylamide gel electrophoresis. Various divalent metal ions were found by a nitrocellulose filter binding assay to effect non-specific interaction of the Fur dimer with DNA with a dissociation constant of 7 × 10−12 M. A much smaller value, 2.5 × 10−17 M, was measured by gel mobility retardation assay for binding of Fur to a DNA fragment containing the operator sequences of the aerobactin promoter.
Similar content being viewed by others
Abbreviations
- Bistris :
-
2-[bis(2-hydroxyethyl)aminol-2-(hydroxymethyl)-propane-1,3-diol
- DMSO :
-
dimethylsulfoxide
- EDTA :
-
ethylenediaminetetraacetate
- Hepes :
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- kb :
-
kilobase
- kDa :
-
kilodalton
- LB :
-
Luria broth
- PMSF :
-
phenylmethylsulfonyl fluoride
- SDS-PAGE :
-
sodium dodecyl sulfate/polyacrylamide gel electrophoresis
- Tris :
-
2-amino-2-hydroxymethylpropane1,3-diol
- TEMED :
-
tetramethylethylenediamine
References
Bagg A, Neilands JB (1985) Mapping of a mutation affecting regulation of iron uptake systems inEscherichia coli K-12. J Bacteriol 161:450–453
Bagg A, Neilands JB (1987a) Molecular mechanism of regulation of siderophore mediated iron assimilation. Microbiol Rev 51:509–518
Bagg A, Neilands JB (1987b) Ferric uptake regulation protein acts as a repressor, employing iron(II) as a cofactor to bind the operator of an iron transport operon inEscherichia coli. Biochemistry 26:5471–5477
Bindereif A, Neilands JB (1983) Cloning of the aerobactin mediated iron assimilation system of plasmid CoIV. J Bacteriol 153:1111–1113
Blackburn S (1970) Protein sequence determinations, methods and techniques. Dekker, New York, pp 25–44
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding. Anal Biochem 72:248–254
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ernst JF, Bennett RL, Rothfield LI (1978) Constitutive expression of the iron enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol 135:928–934
Garibaldi JA, Neilands JB (1956) Formation of iron binding compounds by microorganisms. Nature 177:526–527
Gonzalez N, Wiggs J, Chamberlin MJ (1977) A simple procedure for resolution ofEscherichia coli RNA polymerase holoenzyme from core polymerase. Arch Biochem Biophys 182:404–408
Hantke K (1981) Regulation of ferric ion transport inE. coli. Isolation of a constitutive mutant. Mol Gen Genet 182:288–292
Hantke K (1984) Cloning of the repressor protein gene of iron regulated systems inE. coli K12. Mol Gen Genet 197:337–341
Hendrickson W, Schleif RF (1984) Regulation of theEscherichia coli l-arabinose operon studied by gel electrophoresis DNA binding assay. J Mol Biol 174:611–628
Klig LS, Crawford IP, Yanofsky C (1987) Analysis oftrp repressor-operator interaction by filter binding. Nucleic Acids Res 15:5339–5351
Lorenz V de, Wee S, Herrero M, Neilands JB (1987) Operator sequences of the aerobactin operon of plasmid Co1V-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol 169:2624–2630
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Neilands JB (1981) Microbial iron compounds. Annu Rev Biochem 50:715–731
Neilands JB (1982) Microbial envelope proteins related to iron. Annu Rev Microbiol 36:285–309
Porath J, Carlsson J, Olsson I, Belfrage G (1975) Metal chelate affinity chromatography, a new approach to protein fractionation. Nature 258:598–599
Schaffer S, Hantke K, Braun V (1985) Nucleotide sequence of the iron regulatory genefur. Mol Gen Genet 200:110–113
Straney DC, Crothers DM (1987)Lac repressor is a transient gene-activating protein. Cell 51:699–707
Warner PJ, Williams PH, Bindereif A, Neilands JB (1981) CoIV plasmid specified aerobactin synthesis by invasive strains ofEscherichia coli. Infect Immun 33:540–545
Author information
Authors and Affiliations
Additional information
This work has been supported in part by Grants A104156, PCM 78-12198 and CRCR-1-1633 from the US Public Health Service, National Science Foundation and Department of Agriculture, respectively
Rights and permissions
About this article
Cite this article
Wee, S., Neilands, J.B., Bittner, M.L. et al. Expression, isolation and properties of Fur (ferric uptake regulation) protein ofEscherichia coli K 12. Biol Metals 1, 62–68 (1988). https://doi.org/10.1007/BF01128019
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01128019