Abstract
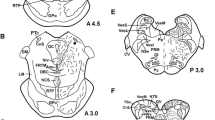
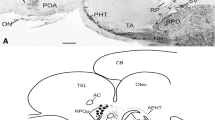
Endozepines are a family of peptides capable of displacing benzodiazepines from their specific binding sites, to which belong the diazepam-binding inhibitor and the octadecaneuropeptide (ODN). This paper reports the distribution of ODN-related peptides, investigated for the first time by immunocytochemistry, in different brain and pituitary regions of the Atlantic hagfish, Myxine glutinosa. Immunoreactive ODN-like material was found in the telencephalon at the level of bundles of different olfactory nerve fibres. Moreover, at the level of the pallium, immunoreactive multipolar neurons were observed in the pars parvocellularis of the stratum griseum superficialis. Similar immunopositive nerve cell bodies were found in the nucleus medialis of the central prosencephalic complex. In the mesencephalon, few immunoreactive neurons lining and contacting the mesencephalic ventricle were detected; such nerve cells could be involved in the regulation of cerebrospinal fluid homeostasis. Dorsally in the mesencephalon, numerous ODN-containing cell bodies were present in the area praetectalis. The rhomboencephalon was immunostained only in the octavolateral area and in the nucleus motorius magnocellularis of the trigeminal nerve. Furthermore, ODN immunoreactivity was also present in the nerve cells of ganglia of the ophthalmic division of the trigeminal nerve complex. The immunocytochemical patterns described here in the brain of M. glutinosa suggest an involvement of ODN-like peptides as neuromodulators in sensory pathways, such as olfactory and visual. Finally, ODN-like substances were localized in discrete populations of adenohypophysial cells and in tanycytes lining the neurohypophyseal walls, suggesting for endozepines a paracrine and/or endocrine control of pituitary hormones release and a neurohormone role respectively. These results could give new insights into the chemioarchitecture of the brain of myxinoids.
Similar content being viewed by others
References cited
Alho E, Costa E, Ferrero P, Fujimoto M, Cosenza-Murphy D, Guidotti A (1985) Diazepam-binding inhibitor: a neuropeptide located in selected neuronal populations of rat brain. Science 229: 179-181.
Alho H, Vaalasti A, Podkletnova I, Rechardt L (1993) Expression of diazepam-binding inhibitor peptide in human skin: an immunohistochemical and ultrastructural study. J Invest Dermatol 101: 800-803.
Ball JA, Burnet PWJ, Fountain BA, Ghatei MA, Bloom SR (1986) Octadecaneuropeptide, benzodiazepine ligand, like immunoreactivity in rat central nervous system, plasma, and peripheral tissues. Neurosci Lett 72: 183-188.
Besman MJ, Yanagibashi K, Lee TD, Kawamura M, Hall PF, Shively JE (1989) Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc Natl Acad Sci USA 86: 4897-4901.
Bovolin P, Schlichting J, Miyata M, Ferrarese C, Guidotti A, Alho H (1990) Distribution and characterization of diazepam binding inhibitor (DBI) in peripheral tissues of rat. Regul Peptides 29: 267-281.
Chen ZW, Agerberth B, Gell K, Andersson M, Mutt V, Ostenson CG, Efendic S, Barros-Soderling J, Persson B, Jornvall H (1988) Isolation and characterization of porcine diazepam-binding inhibitor, a polypeptide not only of cerebral occurrence but also common in intestinal tissues and with effects on regulation of insulin release. Eur J Biochem 174: 239-245.
Chiba A, Honma Y, Oka S (1993) Immunohistochemical localization of neuropeptide Y-like substance in the brain and hypophysis of the brown hagfish, Paramyxine atami. Cell Tissue Res 271: 289-295.
DeBernardi M, Crowe R, Mocchetti I, Shows T, Eddy R, Costa E (1988) Chromosomal localization of the human diazepam binding inhibitor gene. Proc Natl Acad Sci USA 85: 6561-6565.
Donald JA, Vomachka AJ, Evans DH (1992) Immunohistochemical localisation of natriuretic peptides in the brain and hearts of the spiny dogfish Squalus acanthias and the atlantic hagfish Myxine glutinosa. Cell Tissue Res 270: 535-545.
Dores RM, Gorbman A (1990) Detection of met-enkephalin and leu-enkephalin in the brain of the hagfish, Eptatretus stouti, and the lamprey, Petromyzon marinus. Gen Comp Endocrinol 77: 489-499.
Fernholm B (1972) Ultrastructure of the adenohypophysis of Myxine glutinosa. Z Zellforsch Mikrosk Anat 132: 451-472.
Ferrarese C, Appollonio I., Bianchi G, Frigo M, Marzorati C, Pecora N, Perego M, Pierpaoli C, Frattola L (1993) Benzodiazepine receptors and diazepam binding inhibitor: a possible link between stress, anxiety and the immune system. Psychoneuroendocrinology 18: 3-22.
Givalois L, Li S, Pelletier G (1999) Differential involvement of adrenal and gonadal steroids in anterior and intermediate pituitary pro-opiomelanocortin mRNA expression induced by the endogenous benzodiazepine, octadecaneuropeptide, in adult male rats. J Endocrinol 161: 307-316.
Gorbman A, Kobayashi H, Uemura H (1963) The vascularisation of the hypophysial structures of the hagfish. Gen Comp Endocrinol 3: 505-514.
Henderson EN (1972) Ultrastructure of the neurohypophysial lobe of the hagfish Epatretus stouti (Cyclostomata). Acta Zool (Stockh.) 53: 243-266.
Jansen J (1930) The brain of Myxine glutinosa. J Comp Neurol 49: 359-507.
Jirikowski G, Erhart G, Grimmelikhuijzen CJP, Triepel J, Patzner RA (1984) FMRF-amide-like immunoreactivity in brain and pituitary of the hagfish Eptatretus burgeri (Cyclostomata). Cell Tissue Res 237: 363-366.
Johansson O, Hilliges M, Ostenson CG, Sandberg E, Efendic S, Mutt V (1991) Immunohistochemical localization of porcine diazepam-binding inhibitor (DBI) to rat endocrine pancreas. Cell Tissue Res 263: 395-398.
Kavaliers M, Hirst M (1986) An octadecaneuropeptide (ODN) derived from diazepam binding inhibitor increases aggressive interactions in mice. Brain Res 383: 343-349.
Knudsen J, Hojrup P, Hansen HO, Hansen HF, Roepstroff P (1989) Acyl-CoA-binding protein in the rat. Purification, binding characteristics, tissue concentrations and amino acid sequence. Biochem J 26: 513-519.
Kobayashi H, Uemura H (1972) The neurohypophysis of the hagfish, Eptatretus burgeri (Girard). Gen Comp Endocrinol 3 (suppl): 114-124.
Kuhlenbeck H (1975) The Central Nervous System of Vertebrates. Vol. 5, Part I: Derivatives of the Prosencephalon: Diencephalon and Telencephalon. Basel: S. Karger press.
Malagon M, Vallarino M, Tonon MC, Vaudry H (1992a) Localization and characterization of diazepam-binding inhibitor (DBI)-like peptides in the brain and pituitary of the trout (Salmo gairdneri). Brain Res 576: 208-214.
Malagon M, Vaudry H, Vallarino M, Gracia-Navarro F, Tonon MC (1992b) Distribution and characterization of endozepine-like immunoreactivity in the central nervous system of the frog Rana ridibunda. Peptides 13: 99-107.
Marquardt H, Todaro GJ, Shoyab M (1986) Complete amino acid sequences of bovine and human endozepines. J Biol Chem 261: 9727-9731.
Mocchetti I, Einstein R, Brosius J (1986) Putative diazepam binding inhibitor peptide: cDNA clones from rat. Proc Natl Acad Sci USA 83: 7221-7225.
Nishizawa H, Kishida R, Kadota T, Goris RC (1988) Somatotopic organization of the primary sensory trigeminal neurons in the hagfish, Eptatretus burgeri. J Comp Neurol 267: 281-295.
Nozaki M, Gorbman A (1983) Immunocytochemical localization of somatostatin and vasotocin in the brain of the pacific hagfish, Eptatretus stouti. Cell Tissue Res 229: 541-550.
Ohtomi M, Fujii K, Kobayashi H (1989) Distribution of FMR Famide-like immunoreactivity in the brain and neurohypophysis of the lamprey, Lampetra japonica. Cell Tissue Res 256: 581-584.
Olsson R (1959) The neurosecretory hypothalamus-system and the adenohypophysis of Myxine. Z Zellforsch Mikrosk Anat 51: 97-107.
Ostenson CG, Ahren B, Karlsson S, Sandberg E, Efendic S (1990) Effects of porcine diazepam-binding inhibitor on insulin and glucagon secretion in vitro from the rat endocrine pancreas. Regul Peptides 29: 143-151.
Papadopoulos V, Berkovich A, Krueger KE, Costa E, Guidotti A (1991) Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology 129: 1481-1488.
Pestarino M (1994) A possible immunomodulatory role of endozepinelike peptides in a tunicate. Ann NY Acad Sci 712: 365-367.
Rhèaume E, Tonon MC, Smih F, Simard J, Dèsy L, Vaudry H, Pelletier G (1990) Localization of the endogenous benzodiazepine ligand octadecaneuropeptide in the rat testis. Endocrinology 127: 1986-1994.
Ronan M (1988) The sensory trigeminal tract of Pacific hagfish. Primary afferent projections and neurons of the tract nucleus. Brain Behav Evol 32: 169-180.
Slobodyansky E, Guidotti A, Wambebe C, Berkovich A, Costa E (1989) Isolation and characterization of a triakontatetraneuropeptide (TTN), a post-translational product of diazepam binding inhibitor: specific action at the Ro5-4864 recognition sites. J Neurochem 53: 1276-1284.
Tong Y, Tonon MC, Desy L, Nicolas P, Vaudry H, Pelletier G (1990) Localization of the endogenous benzodiazepine receptor ligand octadecaneuropeptide (ODN) and peripheral type benzodiazepine receptors in the rat pituitary. J Neuroendocrinol 2: 189-192.
Tonon MC, Dèsy L, Nicolas P, Vaudry H, Pelletier G (1990) Immunocytochemical localization of the endogenous benzodiazepine ligand octadecaneuropeptide (ODN) in the rat brain. Neuropeptides 15: 17-24.
Tsuneki K, Adachi T, Ishii S, Oota Y (1976) Morphometric classification of neurosecretory granules in the neurohypophysis of the hagfish, Eptatretus burgeri. Cell Tissue Res 166: 145-157.
Wicht H, Nieuwenhuys R (1997) Hagfishes (Myxinoidea). In: Nieuwenhuys R, ten Donkelaar HJ, Nicholson C, eds. The Central Nervous System of Vertebrates, Vol. 1. Heidelberg: Springer Press, pp 497-549.
Wicht H, Northcutt RG (1990) Retinofugal and retinopetal projections in the Pacific hagfish, Eptatretus stouti. Brain Behav Evol 36: 315-328.
Wicht H, Northcutt RG (1994) An immunohistochemical study of the telencephalon and the diencephalon in a myxinoid jawless fish, the Pacific hagfish, Eptatretus stouti. Brain Behav Evol 43: 140-161.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Candiani, S., Augello, A., Oliveri, D. et al. Immunoreactive Endozepine-like Peptides in the Brain and Pituitary of the Atlantic Hagfish, Myxine Glutinosa. Histochem J 32, 415–421 (2000). https://doi.org/10.1023/A:1004091204806
Issue Date:
DOI: https://doi.org/10.1023/A:1004091204806