Abstract
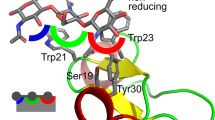
The three-dimensional structure of a glycopeptide, His-Thr*-Ser*-Thr*-Ser*-Ser*-Ser*-Val-Thr-Lys, with 2-acetamido-2-deoxy-α-D-galactose (GalNAc) residues linked to six adjacent amino acids from Thr-10 to Ser-15, was studied by NMR spectroscopy and molecular dynamics (MD) simulations. The hexaglycosylated decapeptide is part of the extracellular domain of human glycophorin A and shows an extended structure of the peptide backbone due to O-glycosylation. Furthermore, each GalNAc residue exhibits one and only one NOE contact from the NHAc proton to the backbone amide proton of the amino acid that the sugar is directly bound to. This indicates a strong preference for the orientation of all GalNAc residues towards the N-terminus. NOE build-up curves were used to determine 42 inter-proton distances that, in connection with φ angles of the peptide backbone obtained from 3J-coupling constants, resulted in constraints for a MD simulation in water. The NMR data and the MD simulations show a preference for an extended backbone structure. The GalNAc residues are located alternatingly on opposite sides of the backbone and reduce the flexibility of the peptide backbone. The conformation of the molecule is relatively rigid and shows a 'wave-type' 3D structure of the peptide backbone within the glycosylation cluster. This new structural element is also supported by the unusual CD spectrum of the glycopeptide.
Similar content being viewed by others
References
Anderson, R.A. and Lovrien, R.E. (1984) Nature, 307, 655–658.
Anderson, R.A. and Marchesi, V.T. (1985) Nature, 318, 295–298.
Batstone-Cunningham, R.L., Hardy, R.E., Daman, M.E. and Dill, K. (1983) Biochim. Biophys. Acta, 746, 1–7.
Butenhof, K.J. and Gerken, T.A. (1993) Biochemistry, 10, 2650–2663.
Coduti, P.L., Gordon, E.C. and Bush, C.A. (1977) Anal. Biochem., 78, 9–20.
de Beer, T., van Zuylen, C.W., Leeflang, B.R., Hård, K., Boelens, R., Kaptein, R., Kamerling, J.P. and Vliegenthart, J.F. (1996) Eur. J. Biochem., 241, 229–242.
Dill, K., Carter, R.D., Lacombe, J.M. and Pavia, A.A. (1985) Carbohydr. Res., 152, 217–228.
El Ouagari, K., Teissie, J. and Benoist, H. (1995) J. Biol. Chem., 45, 26970–26975.
Fukuda, M., Lauffenburger, M., Sasaki, H., Rogers, M.E. and Dell, A.J. (1987) J. Biol. Chem., 262, 11952–11957.
Furthmayr, H. (1978) Supramol. Struct., 9, 195–211.
Furthmayr, H. (1978) Nature, 271, 519–524.
Gasteiger, J. and Marsili, M. (1980) Tetrahedron, 36, 3219–3228.
Klich, G., Paulsen, H., Meyer, B., Meldal, M. and Bock, K. (1997) Carbohydr. Res., 299, 33–48.
Ludvigsen, S., Andersen, K.V. and Poulsen, F.M. (1991) J. Mol. Biol., 217, 731–736.
Marchesi, V.T. (1979) Semin. Hematol., 16, 3–20.
Paulsen, H., Pollex-Kruger, A. and Sinnwell, V. (1991) Carbohydr. Res., 214, 199–226.
Pavia, A.A. and Ferrari, B. (1983) Int. J. Pept. Protein Res., 22, 539–548.
Pieper, J., Ott, K.H. and Meyer, B. (1996) Nat. Struct. Biol., 3, 228–232.
Shogren, R., Gerken, T.A. and Jentoft, N. (1989) Biochemistry, 28, 5525–5536.
Sybyl 6.3 (1996), Force Field Manual, Tripos, St. Louis, MO, pp. 359–371.
Watanabe, K., Matsuda, T. and Sato, Y. (1981) Biochim. Biophys. Acta, 667, 242–250.
Withka, J.M., Wyss, D.F., Wagner, G., Arulanandam, A.R., Reinherz, E.L. and Recny, M.A. (1993) Structure, 1, 69–81.
Wyss, D.F., Dayie, K.T. and Wagner, G. (1997) Protein Sci., 6, 534–542.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schuster, O., Klich, G., Sinnwell, V. et al. 'Wave-type' structure of a synthetic hexaglycosylated decapeptide: A part of the extracellular domain of human glycophorin A. J Biomol NMR 14, 33–45 (1999). https://doi.org/10.1023/A:1008397304851
Issue Date:
DOI: https://doi.org/10.1023/A:1008397304851