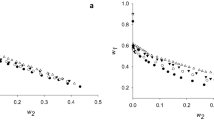
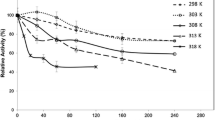
Abstract
An aqueous two-phase system composed by a thermoseparating random copolymer of ethylene oxide/propylene oxide 50/50 (%w/w), Breox, and hydroxypropyl starch – Reppal PES 100 was evaluated for the partitioning of Fusarium solani pisi recombinant cutinase. The effect of several additives on the partitioning of pure cutinase was evaluated. Micelles of sodium dodecanoate provided a ten-fold increase of the partitioning coefficient (K=9) and recovery yields of 60-75%. The phase diagrams of the systems composed of Breox, Reppal and sodium dodecanoate were determined and it was found that in systems with high surfactant concentrations, the binodal was moved to lower polymer concentrations, enabling a two-phase system with 6% (w/w) of each polymer.
Similar content being viewed by others
References
Abbott NL, Blankschtein D and Hatton TA (1990). On protein partitioning in two-phase aqueous polymer systems. Bioseperation 1: 191-225.
Albertsson P-A (1986). Partitioning of Cell Particles and Macromolecules. Wiley, New York.
Alred PA, Kozlowski A, Harris, JM and Tjerneld F (1994) Application of temperature-induced phase partitioning at ambient temperature for enzyme purification. J. Chromatogr. A 659: 289-98.
Ananthapadmanabhan KP (1993) Protein-surfactant interactions. In: Goddard ED and Ananthapadmanabhan KP (ed). Interaction of surfactants with polymers and proteins. CRC Press. Boca Raton, Florida: 319-65.
Baskir JN, Hatton TA and Suter, UW (1989) Protein partitioning in two-phase aqueous polymer systems. Biotechnol. Bioeng. 34: 541-58.
Bodhankar SS and Gaikar VG (1998). Effect of surface active additives on partitioning of proteins and enzymes in poly(ethylene glycol)/Dextran aqueous two-phase systems. J. Chem. Technol. Biotechnol. 73: 251-8.
Bodhankar SS, Harihar V and Gaikar VG (1996) Partitioning of amino-acids in aqueous two-phase system of polyethylene glycol/sodium sulfate in presence of surface active additives. Bioseperation 6: 47-54.
Brackman JC and Engberts JBFN (1994) Interactions between water-soluble nonionic polymers and surfactant aggregates. In: Herb CA and Prod'Homme RK (eds) Structure and Flow in Surfactant Solutions. American Chemical Society pp 337-349. Washington DC: 337–49.
Cabane BJ (1977) Structure of some polymer-surfactant aggregates in water. J. Phys. Chem. 81: 1639-1645.
Carvalho CML, Aires-Barros MR and Cabral JMS (1999) Cutinase: From molecular level to bioprocess development. Biotechnol.Bioeng. 66: 17-34.
Cordes A and Kula MR (1986) Process design for large-scale purification of formate dehydrogenase from Candida boidinii by affinity partition. J. Chromatogr. 376: 375-84.
Creveld LD, Amadei A, van Schaik RC, Pepermans HAM, de Vlieg J and Berendsen HJC (1998) Identification of functional and unfolding motions of cutinase as obtained from molecular dynamics computer simulations. Prot. Struct. Funct. Genet. 33: 253-64.
Cunha MT, Tjerneld F, Cabral JMS and Aires-Barros MR (1998) Effect of electrolytes and surfactants on the thermoseparation of an ethylene oxide-propylene oxide random copolymer in aqueous solution. J. Chromatogr. B 711: 53-60.
Dickinson E (1993) Proteins in solution and at interfaces. In: Goddard ED and Ananthapadmanabhan KP (eds) Interaction of surfactants with polymers and proteins pp 295-317. CRC Press, Boca Raton, Florida
Drouin CM and Cooper DG (1992) Biosurfactants and aqueous two-phase fermentation. Biotechnol. Bioeng. 40: 86-90.
Egmond MR and van Bemmel CJ (1997) Impact of structural information on understanding lipolytic function. In: Rubin B and Dennis E A (ed) Lipases. Academic Press, New York 284: 119-29.
Evans DF (1994). The colloidal domain: where physics, chemistry, biology and technology meet. VCH, New York.
Hart RA, Lester PM, Reifsnyder DH, Ogez JR and Builder SE (1994) Large scale, in situ isolation of periplasmic IGF-I from E. coli. Bio/Technology 12: 1113-1117.
Helenius A and Simons K (1975) Solubilization of membranes by detergents. Biochim. Biophys. Acta 415: 29-70.
Jaeger K-E and Reetz MT (1998) Microbial lipases from versatile tools for biotechnology. Trends Biotechnol. 16: 398-403.
Johansson H-O, Karlström G and Tjerneld F (1997) Effect of solute hydrophobicity on phase behaviour in solutions of thermoseparating polymers. Colloid. Polym. Sci. 257: 458-66.
Johansson H-O, Lundh G, Karlström G and Tjerneld F (1996). Effects of ions in partitioning of serum albumin and lysozyme in aqueous two-phase systems containing ethylene oxide/propylene oxide co-polymers. Biochim. Biophys. Acta 1290: 289-98.
Kolattukudy PE (1984) Cutinases from fungi and pollen. In: Borgström B and Brockman H (eds) Lipases pp 471-504. Elsevier. Amsterdam
Lauwereys M, De Geus P, De Meutter J and Matthyssens G (1991) Cloning, expression and characterization of cutinase, a fungal lipolytic enzyme. In: Alberghina L, Schmid RD and Verger R (eds). Lipases: structure, mechanism and genetic engineering (pp. 243-251). VCH. Weinheim.
Li Y and Dublin PL (1994) Polymer-surfactant complexes. In: Herb CA and Prod'Homme RK (eds) Structure and Flow in Surfactant Solutions (pp 320-336). American Chemical Society. Washington DC.
Martinez C, De Geus P, Lauwereys M, Matthyssens G and Cambillau C (1992) Fusarium solani cutinase is a lipolytic enzyme with a catalytic serine accessible to solvent. Nature 356: 615-618.
Neves MT, Martel P, Petersen EI, Drablos F and Petersen SB (1997) Surface and electrostatics of cutinases. In: Rubin, B. and Dennis EA (eds). Lipases. Academic Press, New York 284: 130-154.
Nikas YJ and Blankschtein D (1994) Complexation of nonionic polymers and surfactants in dilute aqueous solutions. Langmuir 10: 3512-3528.
Petersen SB, Jonson PH, Fojan P, Petersen EI, Petersen MTN, Hansen S, Ishak RJ and Hough E (1998) Protein engineering the surface of enzymes. J. Biotechnol. 66: 11-26.
Piculell L and Lindman B (1992) Association and segregation in aqueous polymer/polymer, polymer/surfactant, and surfactant/surfactant mixtures: Similarities and differences. Adv. Colloid Int. Sci. 41: 149-178.
Pocalyko DJ and Tallman M (1998). Effects of amphipaths on the activity and stability of Fusarium solani pisi cutinase. Enzyme Microb. Technol. 22: 647-651.
Save SV and Pangarkar VG (1995) Behaviour of surfactants in aqueous two phase systems. Bioseperation 5: 27-33.
Sebastião MJ, Cabral JMS and Aires-Barros MR (1996) Improved purification protocol of a Fusarium solani pisi recombinant cutinase by phase partitioning in aqueous two-phase systems of polyethylene glycol and phosphate. Enzyme Microb. Technol. 18: 251-261.
Sivars U, Bergfeldt K, Piculell L and Tjerneld F (1996) Protein partitioning in weakly charged polymer-surfactant aqueous two-phase systems. J. Chromatogr. B 680: 43-53.
Tjerneld F, Berner S, Cajarville A and Johansson G (1986) New aqueous two-phase system based on hydroxypropyl starch useful in enzyme purification. Enzyme Microb.Technol. 8: 417-423.
Veide A, Smeds AL and Enfors S-O (1983). A process for large-scale isolation of β-galactosidase from E.coli. in an aqueous two-phase system. Biotechnol. Bioeng. 25: 1789-1800.
Zhang KW, Karlström G and Lindman B (1992). Phase behaviour of systems of a non-ionic surfactant and a non-ionic polymer in aqueous solution. Colloids and Surfaces 67: 147-55.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teresa Cunha, M., Cabral, J.M., Tjerneld, F. et al. Effect of salts and surfactants on the partitioning of Fusarium solani pisi cutinase in aqueous two-phase systems of thermoseparating ethylene oxide/propylene oxide random copolymer and hydroxypropyl starch. Bioseparation 9, 203–209 (2000). https://doi.org/10.1023/A:1008132108117
Issue Date:
DOI: https://doi.org/10.1023/A:1008132108117