Abstract
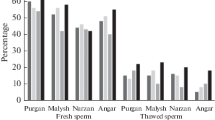
The effects of cryopreservation on two characteristics of human spermatozoa were investigated: the early phases of disturbed plasma membrane function and the activity of enzymes in intact spermatozoa. The membrane function was detected by means of the calcium-dependent binding of fluorescein isothiocyanate (FITC)-conjugated Annexin V to sperm plasma membranes. Annexin V monitors the translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane, which is one of the earliest features of membrane disintegration. For the second aim synthetic fluorogenic substrates for peptidases, proteinases, esterases, elastases and collagenases were applied. These substrates, CellProbe™ reagents consist of different peptide sequences, specific for the enzymes, and a fluorescein- or rhodamine 110-dye moiety. They enter the cells without previous membrane permeabilisation and exhibit fluorescence after cleavage depending on enzyme activity. The number of positive cells and the intensity of the fluorescence were determined by flow cytometric analysis comparing fresh spermatozoa with cryopreserved ones. Thirty-five semen samples collected from 35 donors were cryopreserved using the freezing medium TEST yolk buffer. All specimens showed normal spermiogram parameters. Twenty-five of the samples were used for detection of Annexin V-FITC binding and 10 semen samples for investigations of the intracellular enzymes. The Annexin V-assay applied two fluorescent dyes (Annexin V, AN and propidium iodide, PI) which led to three groups of spermatozoa (a) viable spermatozoa (AN V-negative and PI-negative), (b) dead spermatozoa (AN V-positive and PI-positive) and (c) cells with impaired but integer plasma membrane (AN V-positive and PI-negative). The percentage of vital Annexin V-negative spermatozoa (x ± SEM) decreased significantly (p < 0.001) from fresh spermatozoa (51.6 ± 3.1) to cryopreserved spermatozoa (26.6 ± 2.2%) and was associated with their motility (57.9 ± 1.9% motile fresh spermatozoa vs. 22.6 ± 3.9% motile sperm after cryopreservation). Of the spermatozoa 28.2% were Annexin V-positive before and 44.4% after cryostorage even though they did not bind to PI. Thus, vital spermatozoa showed a disturbed membrane function indicating viability before as well as after cryostorage. Moreover, after cryopreservation the spermatozoal fluorescence increased applying substrates for butyryl esterase (p < 0.05), prolyl-aminopeptidase (p < 0.001) and val-lys-(VK)-cathepsin (p < 0.001). In contrast, the activities of fluorescein diacetate (FDA)- and FDA/sodium fluoride (NAF)-esterase (p < 0.05), ala-ala-pro-val-(AAPV)-elastase (p < 0.001), gly-pro-leu-gly-pro-(GPLGP)-collagenase (p < 0.05) and gly-gly-leu-(GGL)-subtilisin (p < 0.001) decreased after cryopreservation. The substrates for arg-gly-glut-ser-(RGES)-elastase, gly-phenyl-gly-ala-(GFGA)-collagenase and threo-pro-(TP)-cathepsin were not cleaved before as well as after cryostorage. In addition to the known effects of sperm cryopreservation our results showed two further alterations of human ejaculated spermatozoa: (a) disturbed plasma membrane function, which is not detectable by supravital staining and (b) a changed pattern of intracellular enzyme activities.
Similar content being viewed by others
References
Adham IM, Nayernia K and Engel W (1997) Spermatozoa lacking acrosin protein show delayed fertilization. Mol Reprod Develop 46: 370-6
Aitken RJ, Buckingham DW, Carreras A and Irvine DS (1996) Superoxide dismutase in human sperm suspensions: relationship with cellular composition, oxidative stress, and sperm function. Free Radic Biol Med 21: 495-504
Alvarez JG and Storey BT (1993) Evidence that membrane stress contributes more than lipid peroxidation to sublethal cryodamage in cryopreserved human sperm: glycerol and other polyols as sole cryoprotectant. J Androl 14: 199-209
Arienti G, Carlini E, Verdacchi R and Palmerini CA (1997) Transfer of aminopeptidase activity from prostasomes to sperm. Biochim Biophys Acta 1336: 269-74
Barbieri MA, Veisage ML, Paolicchi F, Fornes MW, Sosa MA, Mayorga LS, Bustos-Obregon E and Bertini F (1996) Affinity sites for ?-glucuronidase on the surface of human spermatozoa. Andrologia 28: 327-33
Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P and White JM (1992) Apotential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356: 248-52
Carter J (1996) Live cell functional enzymology using Cell ProbeTM Reagents. Presentation at the XVIII Congress of the International Society for Analytical Cytology, Proceedings of the Congress, p. 1, Rimini, Italy
Chao J, Debasio R, Zhu Z, Giuliano KA and Schmidt BF (1996) Immunofluorescence signal amplification by the enzymecatalysed deposition of a fluorescent receptor substrate (CARD). Cytometry 23: 48-53
Cross NL and Hanks SE (1991) Effects of cryopreservation on human sperm acrosomes. Hum Reprod 6: 1279-83
Davis RO and Katz DF (1992) Standardization and comparability of CASA instruments. J Androl 13: 81-86
DeBaulny BO, Le Vern Y, Kerboeuf D and Maisse G (1977) Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and cryopreserved rainbow trout spermatozoa. Cryobiology 34: 141-9
Evenson DP, Darzynkiewicz Z and Melamed MR (1982) Simultaneous measurement by flow cytometry of sperm cell viability and mitochondrial membrane potential related to cell motility. J Histochem Cytochem 30: 279-80
Ganesh S, Klingel S, Kahle H and Valet G (1995) Flow cytometric determination of aminopeptidase activities in viable cells using fluorogenic rhodamine 110 substrates. Cytometry 20: 334-40
Garner DL and Johnson LA (1995) Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol Reprod 53: 276-84
Glander HJ and Schaller J (1999a) Localisation of enzymes in live spermatozoa by CellProbeTM reagents (preliminary results). Andrologia 31: 37-42
Glander HJ and Schaller J (1999b) Binding of Anexin V to plasma membranes of human spermatozoa: a rapid assay for detection of membrane changes after cryostorage. Mol Human Reprod 5: 109-115
Hamilton M and Steele B (1995) The use of novel fluorogenic enzyme substrates to detect nucleated red blood cells. Clin Chem 41: S239
Hammerstedt RH, Graham JK and Nolan JP (1990) Cryopreservation of mammalian sperm: what we ask them to survive. J Androl 11: 73-88
Haugland RP and Johnson ID (1993) Detecting enzymes in living cells using fluorogenic substrates. J Fluorescence 3: 119-27
Hinsch E, Ponce AA, Hägele W, Hedrich, F, Müller-Schlösser F, Schill WB and Hinsch KD (1997) A new combined in vitro test model for the identification of substances affecting essential sperm functions. Hum Reprod 12: 1673-81
Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG and Zaneveld LJD (1984) Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 70: 219-28
Johnson I (1998) Fluorescent probes for living cells. Histochem J 30: 123-40
Klemm U, Müller-Esterl W and Engel W (1991) Acrosin, the peculiar sperm-specific serine proteinase. Hum Genet 87: 635-41
Klingel S, Rothe G, Kellerman W and Valet G (1994) Flow cytometric determination of cysteine and serine proteinase activities in living cells with rhodamine 110 substrates. Methods Cell Biol 41: 449-59
Kraemer M, Fillion C, Martin-Pont B and Auger J (1998) Factors influencing human sperm kinematic measurements by the Celltrak computer-assisted sperm analysis system. Hum Reprod 13: 611-19
Martin SJ, Reutelingsperger CPM, McGahon, AJ, Rader JA, van Schie RCAA, LaFace DM and Green DR (1995) Earlier distribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exper Med 182: 1545-56
McLaughlin EA, Ford WC and Hull MG (1990) A comparison of the freezing of human semen in the uncirculated vapour above liquid nitrogen and in a commercial semi-programmable freezer. Hum Reprod 5: 719-28
Miyazaki R, Fukuda M, Takeuchi H, Itoh S and Takada M (1990) Flowcytometry to evaluate acrosome-reacted sperm. Arch Androl 25: 243-51
Paasch U and Glander HJ (1997) Optimisation of cryopreservation of subfertile sperm samples: a computer-assisted study. In: Phillips GO, von Versen R, Strong M and Nather A (eds) Advances in Tissue Banking, Vol. 1, pp 233-47. World Scientific, Singapore, New Jersey, London, Hong Kong
Rodriguez-Martinez H, Larsson B and Pertoft H(1997) Evaluation of sperm damage and techniques for sperm clean-up. Reprod Fertil Dev 9: 297-308
Rothe G, Klingel S, Assfalg-Machleidt I, Machleidt W, Zirkelbach C, Banati RB, Mangel WF and Valet G (1992) Flow cytometric analysis of protease activities in vital cells. Biol Chem Hoppe-Seyler 373: 547-54
Sengbusch GV, Couwenbergs C, Kuhner J and Muller U (1976) Fluorogenic substrate turnover in single living cells. Histochem J 8: 341-50
Van Heerde WL, Degroot PG and Reutelingsperger CPM (1995) The complexity of the phospholipid binding protein annexin V. Thromb Haemost 73: 172-9
Vermes I, Haanen C and Reutelingsperger CPM (1995) Anovel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescence labelled annexin V. J Immunol Methods 180: 39-52
Wang Y, Sharma RK and Agarwal A (1997) Effect of cryopreservation and sperm concentration on lipid peroxidation in human semen. Urology 50: 409-13
WHO (1992) World Health Organization Laboratory Manual for Examination of Human Semen and Sperm-Cervical Mucus-Interaction, 3rd edition. Cambridge: Cambridge University Press
Wijchman JG de, Wolf BT and Jager S (1995) Evaluation of a computer-aided semen analysis system with sperm tail detection. Hum Reprod 10: 2090-95
Wilson MJ, Ruhland AR, Pryor JL, Ercole C, Sinha AA, Hensleigh H, Kaye KW, Dawkins HJS, Wasserman NF, Reddy P and Ahmed K (1998) Prostate specific origin of dipeptidylpeptidase IV (CD-26) in human seminal plasma. J Urol 160: 1905-09
Zaneveld LDJ and de Jonge CJ (1991) Mammalian sperm acrosomal enzymes and the acrosome reaction. In: Dunbar BS and O'Rand MG (eds) A Comparative Overview of Mammalian Fertilization. New York: Plenum Press
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Glander, HJ., Schaller, J. Hidden Effects of Cryopreservation on Quality of Human Spermatozoa. Cell Tissue Banking 1, 133–142 (2000). https://doi.org/10.1023/A:1010122800157
Issue Date:
DOI: https://doi.org/10.1023/A:1010122800157