Abstract
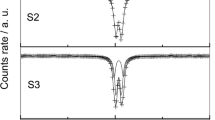
Fe(II)-Fe(III) hydroxysulphite Green Rust 1, GR1(SO3 2−), can be obtained by oxidation of Fe(OH)2 precipitates in aqueous solution and characterised by X-ray diffraction and Mössbauer spectroscopy. In contrast to other Green Rusts (GRs) which then oxidise directly into ferric oxyhydroxides and magnetite, GR1(SO3 2−) first oxidises into a Green Rust two compound, as identified by X-ray diffraction. Mössbauer analysis reveals that this GR2 is the Fe(II)-Fe(III) hydroxysulphate, GR2(SO4 2−). Such an oxidation process, GR1(SO3 2−) → GR2(SO4 2−) confirms that the average oxidation number of Fe increases according to the chemical formulae previously proposed, [FeII 6FeIII 2(OH)16]2+[SO3 · mH2O]2−, and [FeII 4FeIII 2(OH)12]2+[SO4 · nH2O]2− for GR1(SO3 2−) and GR2(SO4 2−) with oxidation numbers of 2.25 and 2.33, respectively. The process implies likely the presence of sulphate ions inherent to the oxidation of sulphite in the solution.
Similar content being viewed by others
References
J.-M.R. Génin, L. Simon and Ph. Refait, ICAME 95 Conf. Proc., I. Ortalli Ed., SIF Bologna, 50 (1996) 51–54.
J.D. Bernal, D.R. Dasgupta and A.L. Mackay, Clay Min. Bull. 4 (1959) 15–30.
L. Ingram and H.F.W. Taylor, Min. Mag. 36 (1967) 465–479.
R. Allmann, Acta Cryst., B24 (1968) 972–977.
A.A. Olowe and J.-M.R. Génin, Corros. Sci. 32 (1991) 965–984.
Ph. Refait and J.-M.R. Génin, Corros. Sci. 34 (1993) 797–819.
H. Drissi, Ph. Refait and J.-M.R. Génin, Hyp. Int. 90 (1994) 395–400.
Ph. Refait and J.-M.R. Génin, ICAME 95 Conf. Proc., I. Ortalli Ed., SIF Bologna, 50 (1996) 55–58.
J.-M.R. Génin, Ph. Refait, L. Simon and S.H. Drissi, Preparation and E h -pH diagrams of Fe(II)-Fe(III) green rust compounds; hyperfine interaction characteristics and stoichiometry of hydroxy-chloride,-sulphate and-carbonate. This issue.
S. Miyata, Clays Clay Miner. 31 (1983) 305–311.
A. Mendiboure and R. Schöllhorn, Rev. Chim. Min. 23 (1986) 819–827.
M. Pourbaix and A. Pourbaix, Rapports Techniques CEBELCOR 159 (1990) RT299.
J.-M.R. Génin, A.A. Olowe, Ph. Refait and L. Simon, Corros. Sci. 38 (1996), 1751–1762.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simon, L., Refait, P. & Génin, JM. Transformation of Fe(II)-Fe(III) Hydroxysulphite into Hydroxysulphate Green Rusts. Hyperfine Interactions 112, 217–222 (1998). https://doi.org/10.1023/A:1010845107110
Issue Date:
DOI: https://doi.org/10.1023/A:1010845107110