Abstract
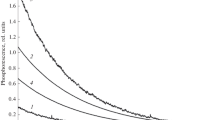
β-Arylpropiophenones do not phosphoresce in homogeneous solution as a result of excited state quenching by the β-aryl group. In the presence of cyclodextrins the parent substrate, β-phenylpropiophenone, shows readily detectable phosphorescence. The complexes show strong phosphorescence after lyophilization to dryness. The phosphorescence intensity of ring-substituted derivatives is strongly dependent upon molecular size and cavity dimensions, suggesting that the β-arylpropiophenones can be used to probe these properties.
Similar content being viewed by others
References
M. L. Bender and M. Komiyama:Cyclodextrin Chemistry, Springer-Verlag, Berlin (1978).
W. Saenger:Angew. Chem., Int., Ed. Engl. 19, 34 (1980).
A. Wishnia and S. J. Lappi:J. Mol. Biol. 82, 77 (1974).
J. Szejtli:Cyclodextrins and their Inclusion Complexes, Akademiai Kiado, Budapest (1982).
R. L., Van Etten, J. F. Sebastian, G. A. Clowes, and M. L. Bender:J. Am. Chem. Soc. 89, 3242 (1967).
S. Scypinski and L. J. Cline Love:Anal. Chem. 56, 322 (1984);
N. J. Turro, J. D. Bolt, Y. Kuroda, and I. Tabushi:Photochem. Photobiol. 35, 69 (1982).
H. L. Casal and J. C. Scaiano:Can. J. Chem. 62, 628 (1984).
H. L. Casal and J. C. Scaiano:Can. J. Chem., in press.
J. C. Netto-Ferreira, W. J. Leigh, and J. C. Scaiano:J. Am. Chem. Soc., in press.
P. J. Wagner, P. A. Kelso, A. E. Kemppainen, A. Haug, and D. R. Graber:Mol. Photochem. 2, 81 (1970).
J. C. Scaiano, M. J. Perkins, J. W. Sheppard, M. S. Platz and R. L. Barcus:J. Photochem. 21, 137 (1983).
F. R. Senti and S. R. Erlander:Carbohydrates (Non-stoichiometric Compounds, Ed. L. Mandelcorn), pp. 568–605, Academic Press, New York, 1964.
J. C. Scaiano, H. L. Casal, and J. C. Netto-Ferreira:ACS Symp. Ser., in press.
A. Habti, D. Keravis, P. Levitz, and H. van Damme:J. Chem. Soc. Faraday Trans. 2 80, 67 (1984).
S. Scypinski and L. J. Cline Love:Anal. Chem. 56, 331 (1984).
Turro, T. Okubo, and G. C. Weed:Photochem. Photobiol. 35, 325 (1982).
N. J. Turro, G. S. Cox, and X. Li:Photochem. Photobiol. 37, 149 (1983).
Author information
Authors and Affiliations
Additional information
Issued as NRCC-23907
Rights and permissions
About this article
Cite this article
Casal, H.L., Netto-Ferreira, J.C. & Scaiano, J.C. Phosphorescence of lyophilized complexes between cyclodextrins andβ-arylpropiophenones. Journal of Inclusion Phenomena 3, 395–401 (1985). https://doi.org/10.1007/BF00657491
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00657491