Abstract
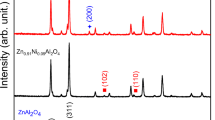
High-quality bulk ZnGa2O4 has been synthesized from equimolar mixtures of ZnO and Ga2O3 by the conventional solid-state method. For the first time, the sample has been characterized in detail to confirm the formation of pure single phase of spinel ZnGa2O4. The formation of ZnGa2O4 has been confirmed by sintering the mixtures of ZnO and Ga2O3 at different temperatures, ranging from 900–1200 °C. It is observed that the single phase of ZnGa2O4 has been formed at and above 1000 °C sintering temperature for 24 h. The crystallinity and phase formation of this single phase has been confirmed by X-ray diffraction. X-ray photoelectron spectroscopic studies have been carried out for bulk ZnGa2O4 sintered at 1000 °C for 24 h which showed 14% Zn, 28% Ga and 58% O, indicating stoichiometric ZnGa2O4. A new parameter, the energetic separation between the Zn 2p3/2 and Ga 2p3/2 peaks, has been used as a sensitive tool to distinguish between a complete formation of ZnGa2O4 compound and a mixture of ZnO and Ga2O3 powders. Surface morphology studies by scanning electron microscopy reveal that the formation of ZnGa2O4 takes place in mosaic rod-like structure. The purity of the compound has also been checked by the energy dispersive X-ray method, indicating the absence of foreign ions and the ratio of zinc to gallium has been calculated and found to be 1 : 2, indicating stoichiometric ZnGa2O4.
Similar content being viewed by others
References
W. S. Hong and L. C. De jonghe, J. Am. Ceram. Soc. 78 (1995) 3217.
S. Itoh, H. Toki, Y. Sato, K. Morimito and T. Kishino, J. Electrochem. Soc. 138 (1991) 1509.
T. Toki, H. Kataoka and S. Itoh, in “Proceedings of the 12th International Display Research Conference”, Hiroshima (1992) p. 412.
L. E. Shea, R. Kdatta and J. J. Brown, J. Electrochem. Soc. 141 (1994) 2198.
I. J. Hsieh, K. T. Chu, C. F. Yu and M. S. Feng, J. Appl. Phys. 76 (1994) 3735.
T. Omata, N. Ueda and K. Ueda, Appl. Phys. Lett. 64 (1994) 1077.
T. Minami, Y. Kuroi and S. Takata, J. Vac. Sci. Technol. A 14 (1996) 1736.
T. K. Tran, W. Park, J. W. Tomm, B. K. Wagner, S. M. Jacobsen and C. J. Summers, J. Appl. Phys. 78 (1995) 5691.
H. M. Manasevit, F. M. Erdman and W. I. Simpson, J. Electrochem. Soc. 118 (1971) 1864.
Hand Book of X-ray Photoelectron Spectroscopy, J. Chastain (ed.) Perkin-Elmer Corporation, Minnesota (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Phani, A.R., Santucci, S., Di Nardo, S. et al. Preparation and characterization of bulk ZnGa2O4. Journal of Materials Science 33, 3969–3973 (1998). https://doi.org/10.1023/A:1004600913743
Issue Date:
DOI: https://doi.org/10.1023/A:1004600913743