Abstract
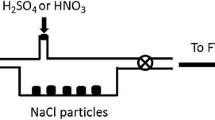
A method for the estimation of the reaction probability of the heterogeneous N2O5+H2O → 2HNO3 reaction using the deposition profile in a laminar flow tube, in which the walls are coated with the condensed aqueous phase of interest, is presented. The production of gas phase nitric acid on the surface followed by its absorption complicates the deposition profiles and hence the calculation of the reaction probability. An estimation of the branching ratio for this process enables a more appropriate calculation to be carried out. Reaction probabilities of N2O5 on substances including some normally constituting atmospheric aerosols, NaCl, NH4HSO4, as well as Na2CO3 are estimated and found to depend on relative humidity and characteristics of the coating used. These fell within the range (0.04–2.0)×10−2.
Similar content being viewed by others
References
Atkinson, R., Winer, A. M., and Pitts Jr. J. N., 1986, Estimation of night-time N2O5 concentration from ambient NO2 and NO3 radical concentrations and the role of N2O5 in night-time chemistry,Atmos. Environ. 20, 331–339.
Finlayson-Pitts, B. J. and Pitts, J. N. Jr., 1986,Atmospheric Chemistry, Wiley, New York.
Heikes, B. G. and Thompson, A. M., 1983, Effects of heterogeneous process on NO3, HONO, and HNO3 chemistry in the troposphere,J. Geophys. Res. 88, 10883–10895.
Johnston, H. S. and Yost, D. M., 1949, The kinetics of the rapid gas reaction between ozone and nitrogen dioxide,J. Chem. Phys. 17, 386–392.
Jones, C. L. and Seinfeld, J. H., 1983, The oxidation of NO2 to nitrate — day and night,Atmos. Environ. 17, 2370–2373.
Kirchner, W., Welter, F., Bongartz, A., Kames, J., Schweighoefer, S., and Schurath, U., 1990, Trace gas exchange at the air/water interface: Measurements of mass accommodation coefficients,J. Atmos. Chem. 10, 427–449.
Miller, J. N. and Miller, J. C., 1998,Statistics for Analytical Chemistry, 2nd edn., Ellis Horwood, Chichester, U.K.
Morris, E. D. Jr. and Niki, H., 1973, Reaction of dinitrogen pentoxide with water,J. Phys. Chem. 77, 1929–1932.
Morris, V. R., Bhatia, S. C., and Hall, J. H., 1987, A study of the gas-phase reaction of NO2 with O3 by matrix isolation infrared spectroscopy,J. Phys. Chem. 91, 3359–3361.
Mozurkewich, M. and Calvert, J. G., 1988, Reaction probability of N2O5 on aqueous aerosols,J. Geophys. Res. 93, 15889–15896.
Msibi, I., Shi, J. P., and Harrison, R. M., 1993, Accommodation coefficient for trace gas uptake using deposition profile measurement in an annular reactor,J. Atmos. Chem. 17, 339–351.
Perry, R. H. and Green, D. W., 1984,Perry's Chemical Engineering Handbook 6th edn., McGraw Hill, New York, pp. 3–285.
Richards, W. L., 1983, Comments on the oxidation of NO2 to nitrate-day and night,Atmos. Environ. 17, 397–402.
Russell, A. G., McCue, K. F., and Cass, G. R., 1985, The dynamics of nitric acid production and the fate of nitrogen oxides,Atmos. Environ. 19, 893–903.
Tuazon, E. C., Atkinson, R., Plum, C. N., Winer, A. M., and Pitts, J. N. Jr., 1983, The reaction of gas phase N2O5 with water vapour,Geophys. Res. Lett. 10, 953–956.
Van Doren, J. M., Watson, L. R., Davidovits, P., Worsnop, D. R., Zahniser, M. S., and Kolb, C. E., 1990, Temperature dependence of the uptake coefficients of HNO3, HCl and N2O5 by water droplets,J. Phys. Chem. 94, 3265–3269.
Van Doren, J. M., Watson, L. R., Davidovits, P., Worsnop, D. R., Zahniser, M. S., and Kolb, C. E., 1991, Uptake of N2O5 and HNO3 by aqueous sulfuric acid droplets,J. Phys. Chem. 95, 1684–1689.
Verhees, P. W. C. and Adema, E. H., 1985, The NO2-O3 system at sub-ppm concentrations: Influence of temperature and relative humidity,J. Atmos. Chem. 2, 387–403.
Wu, C. H., Morris Jr. E. D., and Niki, H., 1973, The reaction of nitrogen dioxide with ozone,J. Phys. Chem. 77, 2507–2511.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Msibi, I.M., Li, Y., Shi, J.P. et al. Determination of heterogeneous reaction probability using deposition profile measurement in an annular reactor: Application to the N2O5/H2O reaction. J Atmos Chem 18, 291–300 (1994). https://doi.org/10.1007/BF00696784
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00696784