Abstract
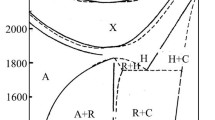
The phase diagram of the La-S-O system at 1073 K was established with the vacuum seal technique. Six phases exist at this temperature: La2O3 (B-type), LaS2, La2S3, La2O2SO4, La2O2S and La2O2S2. The thermodynamic functions for the reaction La2O2SO4=La2O3+SO2+1/2 O2 were determined by using the emf method at temperatures from 1123 to 1373 K. The mechanisms of the oxidation reactions in the La-S-O system under different partial pressures of oxygen (−4.4 < log\(P_{O_2 }\) <−0.7) were also investigated by means of DTA, TG and powder X-ray diffractometry.
Zusammenfassung
Das Phasendiagramm des La-S-O-Systems bei 1073 K wurde bestimmt. Bei dieser Temperatur liegen 6 Phasen vor, und zwar La2O3 (B-Typ), LaS2, La2S3, La2O2SO4, La2O2S und La2O2S2. Für die Reaktion La2O2SO4=La2O3+SO2+1/2 O2 wurden die thermodynamischen Funktionen im Temperaturbereich von 1123–1373 K nach der EMF-Methode bestimmt. DTA, TG und Pulver-Röntgendiffraktometrie wurden zur Untersuchung der Mechanismen der im La-S-O-System verlaufenden Oxydationsreaktionen herangezogen, wobei der Sauerstoffpartialdruck in den Grenzen von − 4.4 < log\(p_{O_2 }\) < <-0.7 variiert wurde.
Резюме
С помощью вакуумной и золированной техник и была установлена фаз овая диаграмма системы La-S-O п ри 1073 К. При этой темпера туре существует шесть фаз: La2O3 (Б-типа), LaS2, La2S3, La2O2SO4, La2O2S и La2O2S2. Используя метод э. д. с., были определены термодинамические п араметры реакции La2O2SO4=La2O3+ SO2+1/2 О2 в температурном интербале 1123–1373. С помощь ю ДТА, ТГ и порошковой рентгенодиффрактом етрии исследованы механиз мы реакций в системе La-S-O б области парциальных давлени й кислорода −4.4 < log\(P_{O_2 }\)< −0.7.
Similar content being viewed by others
References
P. N. Mehrotra, G. V. Chandrashekar, C. N. Rao andE. C. Subbarao, Trans. Faraday Soc., 62 (4) (1966) 3586.
A. E. Solovéva, A. M. Gavrish andE. I. Zoz, Zh. Neorg. Khim., 19 (1974) 1446.
M. W. Nathans andW. W. Wendlandt, J. Inorg. Nucl. Chem., 24 (1962) 869.
A. A. Grizik, N. G. Abdullina andN. M. Garifdzhanova, Zh. Neorg. Khim., 19 (9) (1974) 2586.
N. A.Toropov, V. P.Barzakovskii, V. V.Lapin and N. N.Kurtseva, Translated by J. Schmorak and R. Kondor, “Handbook of Phase Diagrams of Silicate Systems, Metal Oxygen Compounds in Silicate Systems, Vol. II”, Israel Program for Scientific Translations, 1970.
A. W. Sleight andC. T. Prewitt, Inorganic Chemistry, 7 (11) (1968) 2282.
L. F. Vereshchagin, A. A. Eliseev, G. M. Kuzmicheva, V. V. Evdokimova, V. I. Novokshonov andO. P. Fiulkovskii, Zh. Neorg. Khim., 20 (6) (1975) 1466.
P. Besançon andP. Laruelle, Compt. Rend. Acad. Sci. Paris, Série C, 268 (1969) 48.
P. Besançon andM. Guittard, Compt. Rend. Acad. Sci. Paris, Série C, 273 (1971) 1348.
P. Besançon, J. Solid State Chem., 7 (1973) 232.
P. Besançon, D. Carré, M. Guittard andJ. Flahaut, Compt. Rend. Acad. Sci. Paris, Série C, 271 (1970) 679.
P. Besançon, D. Carré andP. Laruelle, Acta Cryst., B29 (1973) 1064.
J. Flahaut andE. Attal, Compt. Rend. Acad. Sci. Paris, 238 (1954) 682.
P. J. Dugué, D. Carré andM. Guittard, Acta Cryst. B34 (1978) 403.
S. Benazeth, M. Guittard andJ. Flahaut, J. Solid State Chem., 37 (1981) 44.
I.Barin, O.Knacke and O.Kubaschewski, Thermochemical Properties of Inorganic Substances (supplement), Springer-Verlag, 1977.
I.Barin and O.Knacke, Thermochemical Properties of Inorganic Substances, Springer-Verlag, 1973.
V. I. Laptev andN. P. Sochchin, Zh. Fiz. Khim., 48 (1974) 2156.
H. H. Kellog, Trans. Met. Soc. AIME, 230 (1964) 1622.
W. W. Wendlandt andT. D. George, J. Inorg. Nucl. Chem., 19 (1961) 245.
W. W. Wendlandt, J. Inorg. Nucl. Chem., 7 (1958) 51.
J. A. Fahey, Proc. Rare Earth Res. Conf., 12th (2) (1976) 762.
D. R.Still and H.Prophet, “JANAF Thermodynamic Tables, Second Edition”, Horikoshi Laboratory, 1971.
M. N. Verkhovets, A. A. Kamarzin andV. V. Sokolov, Izv. Sib. Akad. Nauk, Ser. Khim. Nauk, (1973) (6) 125.
T. Toide, T. Utsunomiya, M. Sato, Y. Hoshino, T. Hatano andY. Akimoto, Nippon Kagakukai Shi, 12 (1972) 2438.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kitazawa, Y., Kunimoto, Y., Wakihara, M. et al. Phase equilibria and oxidation mechanisms in the LA-S-O system. Journal of Thermal Analysis 25, 279–290 (1982). https://doi.org/10.1007/BF01912953
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01912953