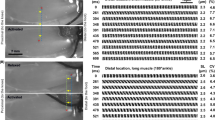
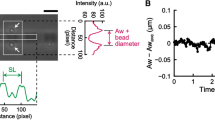
Abstract
The time course of shortening was investigated in the single sarcomere, the smallest contractile unit that retains natural structure. We projected the striation patterns of single bumblebee flight-muscle myofibrils onto a linear photodiode array, which was scanned periodically to produce repetitive traces of intensity vs. position along the array. Sarcomere length was taken as the span between adjacent A-band or Z-line centroids. When myofibrils were ramp-released by a motor, individual sarcomeres shortened in steps punctuated by pauses. The single sarcomere-shortening trace was consistently stepwise both in activated and relaxed specimens. Although step size was variable, the size distribution showed a signature-like feature: the histogram comprised distinct peaks that were spaced quasi-regularly. In the activated myofibrils the interpeak separation corresponded to 2.71 nm per half-sarcomere. This value is equal to the linear advance of actin subunits along the thin filament. Thus, actin filaments translate over thick filaments by steps that may be integer multiples of the actin-subunit spacing.
Similar content being viewed by others
References
Altringham J, Bottinelli R and Lacktis J (1984) Is stepwise sarcomere shortening an artifact? Nature 307: 653–655.
Baba SA (1979) Regular steps in bending cilia during the eective stroke.Nature 282: 717–720.
Bartoo ML, Popov VI, Fearn L and Pollack GH (1993) Active tension generation in isolated skeletal myofibrils. J Muscle Res Cell Motil 14: 498–510.
Bhushan B, Israelachvili J and Landman U (1995) Nanotribology: friction, wear and lubrication at the atomic scale. Nature 374: 607–616.
Bullard B and Leonard K (1996) Modular proteins of insect muscle. Adv Biophys 33: 211–221.
Burton K and Huxley A (1995) Identification of source of oscillations in apparent sarcomere length measured by laser diraction. Biophys J 68: 2429–2443.
Delay MJ, Ishide N, Jacobsen R, Pollack GH and Tirosh R (1981) Stepwise sarcomere shortening: analysis by high–speed cinemicro– graphy. Science 213: 1523–1525.
Diestler D, Schoen M and Cushman J (1993) On the thermo– dynamic stability of confined thin films under shear. Science 262: 545–547.
Erickson HP (1994) Reversible unfolding of fibronectin type III and immunoglobulin domains provides the structural basis for stretch and elasticity of titin and fibronectin. Proc Natl Acad Sci USA 91: 10114–10118.
Fauver M, Dunaway D, Lilienfeld D, Craighead HG and Pollack GH (1999) Microfabricated cantilevers for measurement of subcellular and molecular forces. IEEE Trans Biomed Eng, in press.
Gee M, McGuiggan P and Israelachvili JN (1990) Liquid to solidlike transitions of molecularly thin films under shear. J Chem Phys 93: 1895–1906.
Gelles J, Schnapp BJ and Sheetz MP (1988) Tracking kinesin–driven movements with nanometre–scale precision. Nature 331: 450–453.
Goldman Y and Simmons R (1984) Control of sarcomere length in skinned muscle fibers of Rana temporia during mechanical tran– sients. J Physiol 184: 497–518.
Granzier H, Myers J and Pollack GH (1987) Stepwise shortening of muscle fiber segments. J Muscle Res Cell Motil 8: 242–251.
Granzier H, Mattiazzi A and Pollack GH (1990) Sarcomere dynamics during isotonic velocity transients in single frog muscle fibers. Am J Physiol (Cell) 259: C266–C278.
Hua W, Young EC, Fleming ML and Gelles J (1997) Coupling of kinesin steps to ATP hydrolysis. Nature 388: 390–393.
Huxley AF and Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233: 533–538.
Improta S, Politou AS and Pastore A (1996) Immunoglobulin–like modules from titin I–band: extensible components of muscle elasticity. Structure 4: 323–337.
Israelachvili J, McGuiggan P, Gee M, Homola A, Robbins M and Thompson P (1990) Liquid dynamics in molecularly thin films. J Phys Condens Matter 2: SA89–SA98.
Jacobsen RC, Tirosh R, Delay MJ and Pollack GH (1983) Quantized nature of sarcomere shortening steps. J Muscle Res Cell Motil 4: 529–542.
Kojima H, Muto E, Higuchi H and Yanagida T (1997) Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys J 73: 2012–2022.
Kuo SC, Gelles J, Steuer E and Sheetz MP (1991) A model for kinesin movement from nanometer–level movements of kinesin and cytoplasmic dynein and force measurements. J Cell Sci Suppl 14: 135–138.
Labeit S and Kolmerer B (1995) Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270: 293–296.
Ling GN (1984) In Search of the Physical Basis of Life. Plenum, New York.
Maruyama K, Toshitada Y, Yoshidomi H, Sawada H and Kikuchi M (1984) Molecular size and shape of b–connectin, an elastic protein of striated muscle. J Biochem 95: 1423–1493.
Millikan RA (1947) Electrons (+and ÿ), Protons, Photons, Neutrons, Mesotrons, and Cosmic Rays, 2nd ed. University of Chicago Press, Chicago.
Molloy JE, Burns JE, Kendrick–Jones J, Tregear RT and White DC (1995) Movement and force produced by a single myosin head. Nature 378: 209–212.
Politou AS, Thomas DJ and Pastore A (1995) The folding and stability of titin immunoglobulin–like modules with implications for the mechanism of elasticity. Biophys J 69: 2601–2610.
Pollack GH, Iwazumi T, ter Keurs HEDJ and Shibata EF (1977) Sarcomere shortening in straited muscle occurs in stepwise fashion. Nature 268: 757–759.
Pollack GH (1990) Muscles and Molecules: Uncovering the Principles of Biological Motion. Ebner and Sons, Seattle, WA.
Pollack GH (1996) Phase transitions and the molecular mechanism of contraction. Biophys Chem 59: 315–328.
Prochniewicz E, Zhang Q, Janmey PA and Thomas DD (1996) Cooperativity in F–actin: binding of gelsolin at the barbed end aects structure and dynamics of the whole filament. J Mol Biol 260: 756–766.
Rief M, Gautel M, Oesterhelt F, Fernandez JM and Gaub HE (1997) Reversible unfolding of individual titin immunoglobulin domains by AFM. Science 276: 1109–1112.
Rudel R and Zite–Frenczy F (1979) Do laser diraction studies on striated muscle indicate stepwise sarcomere shortening? Nature 278: 573–575.
Saito K, Aoki T, Aoki T and Yanagida T (1994) Movement of single myosin filaments and myosin step size on an actin filament suspended in solution by a laser trap. Biophys J 66: 769–777.
Schnitzer M and Block SM (1997) Kinesin hydrolyses one ATP per 8– nm step. Nature 388: 386–390.
Schutt C and Lindberg U (1992) Actin as the generator of contraction during muscle contraction. Proc Natl Acad Sci USA 89: 319–323.
Squire JA (1981) The Structural Basis of Muscular Contraction. Plenum Press, New York.
Svoboda K, Schmidt CF, Schnapp BJ and Block SM (1993) Direct observation of kinesin stepping by optical trapping interferometry. ]Nature 365: 721–727.
Tameyasu T (1994) Oscillatory contraction of single sarcomere in single myofibril of glycerinated, striated adductor muscle of scallop. Jpn J Physiol 44: 295–318.
Tameyasu T, Toyoki T and Sugi H (1985) Non–steady motion in unloaded contractions of single frog cardiac cells. Biophys J 48: 461–465.
Thompson P and Robbins M (1990) Origin of stick–slip motion in boundary lubrication. Science 250: 792–794.
Toride M and Sugi H (1989) Stepwise sarcomere shortening in locally activated frog skeletal muscle fibers. Proc Jpn Acad 65(B3): 49–52.
Tourovskaya A and Pollack GH (1998) Stepwise length changes during stretch in single sarcomeres of single myofibrils. Biophys J 74: A153.
Trinick J, Knight P and Whiting A (1984) Purification and properties of native titin. J Mol Biol 180: 331–356.
Trombitás K and Pollack GH (1995) Actin filaments in muscle cells move collectively. Cell Motil Cytoskel 32: 145–150.
Trombitás K, Baatsen P and Pollack GH (1988) I–bands of striated muscle contain lateral struts. J Ultrastruct Mol Str Res 100: 13–30.
Tskhovrebova L, Trinick J, Sleep JA and Simmons RM (1997) Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature 387: 308–312.
Wang SM and Greaser ML (1985) Immunocytochemical studies using a monoclonal antibody to bovine cardiac titin on intact and extracted myofibrils. J Cell Biol 107: 1075–1083.
Warshaw D (1996) The in vitro motility assay: a window into the myosin molecular motor. News Physiol Sci 11: 1–6.
Yang P, Tameyasu T and Pollack GH (1998) Stepwise dynamics of connecting–filaments in single myofibrillar sarcomeres. Biophys J 74: 1473–1483.
Yoshizawa H and Israelachvili J (1993) Fundamental mechanisms of interfacial friction. 2. Stick–slip friction of spherical and chain molecules. J Phys Chem 97: 11300–11313.
Zocchi G (1997) Proteins unfold in steps. Proc Natl Acad Sci USA 94: 10647–10651.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blyakhman, F.A., Shklyar, T. & Pollack, G.H. Quantal length changes in single contracting sarcomeres. J Muscle Res Cell Motil 20, 529–538 (1999). https://doi.org/10.1023/A:1005590401721
Issue Date:
DOI: https://doi.org/10.1023/A:1005590401721