Abstract
The biosynthetic pathway to 4-hydroxybenzoate (4HB), a precursor of the naphthoquinone pigment shikonin, was modified in Lithospermum erythrorhizon hairy root cultures by introduction of the bacterial gene ubiC. This gene of Escherichia coli encodes chorismate pyruvate-lyase (CPL), an enzyme that converts chorismate into 4HB and is not normally present in plants. The ubiC gene was fused to the sequence for a chloroplast transit peptide and placed under control of a constitutive plant promoter. This construct was introduced into L. erythrorhizon by Agrobacterium rhizogenes-mediated transformation.
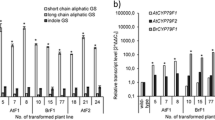
The resulting hairy root cultures showed high CPL activity. 4HB produced by the CPL reaction was utilized for shikonin biosynthesis, as shown by in vivo inhibition of the native pathway to 4HB with 2-aminoindan-2-phosphonic acid (AIP), an inhibitor of phenylalanine ammonia-lyase. A feeding experiment with [1,7-13C2]shikimate showed that in the absence of AIP the artificially introduced CPL reaction contributed ca. 20% of the overall 4HB biosynthesis in the transgenic cultures. ubiC transformation did not lead to a statistically significant increase of shikonin formation, but to a 5-fold increase of the accumulation of menisdaurin, a nitrile glucoside which is presumably related to aromatic amino acid metabolism.
Similar content being viewed by others
References
Baulcombe DC, Saunders GR, Bevan MW, Mayo MA, Harrison BD: Expression of biologically active viral satellite RNA from the nuclear genome of transformed plants. Nature 321: 446–449(1986).
Cho H, Heide L, Floss HG: Synthesis of D-(-)-[1,7–13C2]shikimic acid. J Labelled Compd Radiopharm 31: 589–594 (1992).
Colliver SP, Morris P, Robbins MP: Differential modification of flavonoid and isoflavonoid biosynthesis with an antisense chalcone synthase construct in transgenic Lotus corniculatus. Plant Mol Biol 35: 509–522(1997).
Fujita Y, Hara Y, Ogino T, Suga C: Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. I. Effects of nitrogen sources on the production of shikonin derivatives. Plant Cell Rep 1: 59–60(1981).
Fujita Y, Hara Y, Suga C, Morimoto T: Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. II. A new medium for the production of shikonin derivatives. Plant Cell Rep 1: 61–63(1981).
Gaisser S, Heide L: Inhibition and regulation of shikonin biosynthesis in suspension cultures of Lithospermum. Phytochemistry 41: 1065–1072(1996).
Gatz C, Frohberg C, Wendenburg R: Stringent repression and homogeneous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J 2: 397–404(1992).
Hamill JD, Parr AJ, Michael JC, Rhodes MJC, Robins RJ, Walton NJ: New routes to plant secondary products. Bio/technology 5: 800–804(1987).
Hayashi M: Pharmacological studies on crude plant drugs, Shikon and Tooki. III. Effect of topical application of the ether extracts and Shiunko on inflammatory reaction. Folia Pharmacol Japon 73: 205–214(1977).
Heide L, Floss HG, Tabata M: Incorporation of shikimic acid into p-hydroxybenzoic acid in Lithospermum erythrorhizon cell cultures. Phytochemistry 28: 2643–2645(1989).
Heide L, Nishioka N, Fukui H, Tabata M: Enzymatic regulation of shikonin biosynthesis in Lithospermum erythrorhizon cell cultures. Phytochemistry 28: 1873–1877(1989).
Höfgen R, Willmitzer L: Storage of competent cells for Agrobacterium transformation. Nucl Acids Res 16: 9877 (1988).
Inouye H, Ueda S, Inoue K, Matsumura H: Biosynthesis of shikonin in callus cultures of Lithospermum erythrorhizon. Phytochemistry 18: 1301–1308(1979).
Lange BM, Severin K, Bechthold A, Heide L: Regulatory role of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase for shikonin biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Planta 204: 234–241 (1998).
Li S-M, Wang Z-X, Wemakor E, Heide L: Metabolization of the artificial secondary metabolite 4-hydroxybenzoate in ubiCtransformed tobacco. Plant Cell Physiol 38: 844–850(1997).
Lodhi AH, Bongaerts RJM, Verpoorte R, Coomber SA, Charlwood BV: Expression of bacterial isochorismate synthase (EC 5.4.99.6) in transgenic root cultures of Rubia peregrina. Plant Cell Rep 16: 54–57(1996).
Löscher R, Heide L: Biosynthesis ofp-hydroxybenzoate from p-coumarate and p-coumaroyl-coenzyme A in cell-free extracts of Lithospermum erythrorhizon cell cultures. Plant Physiol 106: 271–279(1994).
Murashige T, Skoog F: A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497(1962).
Nichols BP, Green JM: Cloning and sequencing of Escherichia coli ubiC and purification of chorismate lyase. J Bact 174: 5309–5316(1992).
Papageorgiou VP: Naturally occuring isohexenylnaphthazarin pigments: a new class of drugs. Planta Med 38: 193–203 (1980).
Sankawa U, Ebizuka Y, Miyazaki T, Isomura Y, Otsuka H, Shibata S, Inomata M, Fukuoka F: Antitumor activity of shikonin and its derivatives. Chem Pharm Bull 25: 2392–2395(1977).
Schmid HV, Zenk MH: p-Hydroxybenzoic acid and mevalonic acid as precursors of the plant naphthoquinone alkannin. Tetrahedron Lett 44: 4151–4155(1971).
Shimomura K, Sudo H, Saga H, Kamada H: Shikonin production and secretion by hairy root cultures of Lithospermum erythrorhizon. Plant Cell Rep 10: 282–285(1991).
Siebert M, Bechthold A, Melzer M, May U, Berger U, Schröder G, Schröder J, Severin K, Heide L: Ubiquinone biosynthesis: Cloning of the genes coding for chorismate pyruvate-lyase and 4-hydroxybenzoate octaprenyl transferase from Escherichia coli. FEBS Lett 307: 347–350(1992).
Siebert M, Severin K, Heide L: Formation of 4-hydroxybenzoate in Escherichia coli: characterization of the ubiC gene and its encoded enzyme chorismate pyruvate-lyase. Microbiology 140: 897–904(1994).
Siebert M, Sommer S, Li S-M, Wang Z-X, Severin K, Heide L: Genetic engineering of plant secondary metabolism. Accumulation of 4-hydroxybenzoate glucosides as a result of the expression of the bacterial ubiC gene in tobacco. Plant Physiol 112: 811–819(1996).
Sommer S, Severin K, Camara B, Heide L: Intracellular localization of geranylpyrophosphate synthase from cell cultures of Lithospermum erythrorhizon. Phytochemistry 38: 623–627 (1995).
Sommer S, Siebert M, Bechthold A, Heide L: Specific induction of secondary product formation in transgenic plant cell cultures by use of an inducible promoter. Plant Cell Rep 17: 891–896(1998).
Sosa A, Winternitz F, Wylde R, Pavia AA: Structure of a cyanoglucoside of Lithospermum purpureo-caeruleum. Phytochemistry 16: 707–709(1977).
Tang W, Eisenbrand G: Lithospermum erythrorhizon Sieb. et Zucc. Springer Verlag, Berlin/Heidelberg/New York (1992).
Tabata M, Fujita Y: Production of shikonin by plant cell cultures. In: Day P, Zaitlin M, Hollaender A (eds), Biotechnology in Plant Science, pp. 207–218. Academic Press, Orlando, FL (1985).
Takahashi K, Matsuzawa S, Takani M: Studies on the constituents of medicinal plants. XX. The constituent of the vines of Menispermum dauricum DC. Chem Pharm Bull 26: 1677–1681(1978).
Tanaka Y, Odani T: Pharmacodynamic study on 'shiunko'. I. Antibacterial effect of 'shiunko'. Yakugaku Zasshi 92: 525–530 (1972).
Tsukada M, Tabata M: Intracellular localization and secretion of naphthoquinone pigments in cell cultures of Lithospermum erythrorhizon. Planta Med 50: 338–340(1984).
Yamaga Y, Nakanishi K, Fukui H, Tabata M: Intracellular localization of p-hydroxybenzoate geranyltransferase, a key enzyme involved in shikonin biosynthesis. Phytochemistry 32: 633–636(1993).
Yazaki K, Fukui H, Tabata M: Accumulation of p-O-_-Dglucosylbenzoic acid and its relation to shikonin biosynthesis in Lithospermum cell cultures. Phytochemistry 25: 1629–1632 (1986).
Yazaki K, Inushima K, Kataoka M, Tabata M: Intracellular localization of UDPG:p-hydroxybenzoate glucosyltransferase and its reaction product in Lithospermum cell cultures. Phytochemistry 38: 1127–1130(1995).
Zon J, Amrhein N: Inhibitors of phenylalanine ammonialyase: 2-aminoindan-2-phosphonic acid and related compounds. Liebigs Ann Chem 625–628(1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sommer, S., Köhle, A., Yazaki, K. et al. Genetic engineering of shikonin biosynthesis hairy root cultures of Lithospermum erythrorhizon transformed with the bacterial ubiC gene. Plant Mol Biol 39, 683–693 (1999). https://doi.org/10.1023/A:1006185806390
Issue Date:
DOI: https://doi.org/10.1023/A:1006185806390