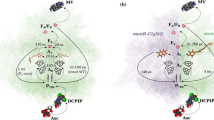
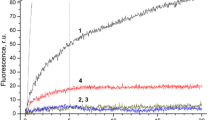
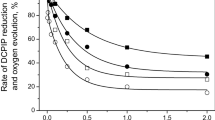
Abstract
The inhibitory effect of Zn2+ on photosynthetic electron transport was investigated in native and CaCl2-treated (depleted in extrinsic polypeptides) Photosystem II (PS II) submembrane preparations. Inhibition of 2,6-dichlorophenolindophenol photoreduction by Zn2+ was much stronger in protein-depleted preparations in comparison to the native form. It was found that Ca2+ significantly reduced the inhibition in the native PS II preparations, as did Mn2+ in a combination with H2O2 in the protein-depleted counterparts. No other tested monovalent or divalent cations could replace Ca2+ or Mn2+ in the respective experiments. Diphenylcarbazide could partially relieve (40–45%) the inhibition in both types of preparations. The above indicates the presence of an active Zn2+ inhibitory site on the donor side of PS II. However, neither Ca2+ nor Mn2+ could completely prevent inhibition by high concentrations of Zn2+ (>1 mM). We propose that elevated levels of Zn2+ strongly perturb the conformation of the PS II core complex and might also affect the acceptor side of the photosystem.
Similar content being viewed by others
Abbreviations
- PMSF:
-
phenylmethanesulfonyl fluoride
- MES:
-
2-(N-morpholino)ethane sulphonic acid
- Chl:
-
chlorophyll
- PS II:
-
Photosystem II
- DCIP:
-
2,6-dichlorophenolindophenol
- DPC:
-
sym-diphenylcabazide
- DCBQ:
-
2,5-dichlorobenzoquinone
References
Agrawala SC, Bishit SS and Sharma CP (1977) Relative effectiveness of some heavy metals in producing toxicity and symptoms of iron-deficiency in barley. Can J Bot 55: 1299–1307
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24: 1–15
Baker NR, Fernyhough P and Meek IT (1982) Light dependent inhibition of photosynthetic electron transport by zinc. Physiol Plant 56: 217–222
Berthold DA, Babcock GT and Yocum CF (1981) A highly resolved oxygen evolving Photosystem II preparation from spinach thylakoid membranes. EPR and electron transport properties. Febs Lett 134: 231–234
Buchauer MJ (1973) Contamination of soil and vegetation near a zinc smelter by zinc, cadmium, copper and lead. Environ Sci Technol 7: 131–135
Carpentier R, Larue B and Leblanc RM (1984) Photoacoustic spectroscopy of Anacystic nidulans III. Detection of photosynthetic activities. Arch Biochem Biophys 228: 534–543
Davies AG and Sleep JA (1979) Photosynthesis in some British coastal water may be inhibited by zinc pollution. Nature 277: 292–293
Dismukes CG (1988) The spectroscopically derived structure of the Mn site for photosynthetic H2O oxidation and a proposal for the protein-binding sites for Ca2- and Mn2+. Chemica Scripta 28A, 99–104
Dunahay TD, Staehelin LA, Seibert M, Ogilvie PD and Berg SP (1984) Structural, biochemical and biophysical characterization of four oxygen-evolving Photosystem II preparations from spinach. Biochim Biophys Acta 764: 179–193
Ghanotakis DF, Topper JN and Yocum CF (1984) Structural organization of the oxidizing site of Photosystem II. Exogenous Photosystem II membranes depleted of 17 and 23 kDa polypeptides. Biochim Biophys Acta 767: 524–531
Homann PH (1987) The relations between the chloride, calcium and polypeptide requirements of photosynthetic water oxidation. J Bioenerg Biomemb 19: 105–123
Homann PH (1988) Chloride relations of PS II membrane preparations depleted of, and resupplied with, their 17 and 23 kDa extrinsic polypeptides. Photosynth Res 15: 205–220
Inoue H and Nishimura M (1971) Electron flow from hydrogen peroxide in Photosystem II-catalyzed oxidation-reduction reactions of spinach chloroplast fragments. Plant Cell Physiol 12: 737–747
Inoue H and Wada T (1987) Requirement of manganese for electron donation of hydrogen peroxide in Photosystem II reaction center complex. Plant Cell Physiol 28: 767–773
Inoue H, Akahori H and Noguchi M (1987) Activation of electron donation from hydrogen peroxide by manganese in non-oxygen evolving Photosystem II particles. Plant Cell Physiol 28: 1339–1343
Miller M and Cox RP (1983) Effect of Zn2+ on photosynthetic oxygen evolution and chloroplast manganese. FEBS Lett 155: 331–333
Mohanty N, Vass I and Demeter S (1989) Impairment of Photosystem II activity at the level of secondary quinone electron acceptor in chloroplasts treated with cobalt, nickel and zinc ions. Physiol Plant 76: 386–390
Nakatani HY (1984) Photosynthetic oxygen evolution does not require the participation of polypeptides of 16 and 24 kDa. Biochem Biophys Res Comm 120: 299–304
Ono T-A and Inoue Y (1983) Mn-preserving extraction of 33-, 24- and 16-kDa proteins from O2-evolving PS II particles by divalent salt washing. FEBS Lett 164: 255–260
Pan RL and Izawa S (1979) Photosystem II energy coupling in chloroplasts with H2O2 as electron donor. Biochim Biophys Acta 547: 311–319
Rashid A and Carpentier R (1989) CaCl2 inhibition of H2O2 electron donation to Photosystem II in submembrane preparations depleted in extrinsic polypeptides. FEBS Lett 258: 331–334
Sandusky PO and Yocum CF (1988) Hydrogen peroxide oxidation catalized by chloride-depleted thylakoid membranes. Biochim Biophys Acta 936: 149–156
Schröder WP and Åkerlund H-E (1986) H2O2 accessibility to the Photosystem II donor side in protein-depleted insideout thylakoids measured as flash-induced oxygen production. Biochim Biophys Acta 848: 359–363
Schrotri CK, Rathore VS and Mohanty P (1981) Studies on photosynthetic electron transport, photophosphorylation and CO2 fixation in Zn2+ deficient leaf cells of Zea mays. J Plant Nutri 3: 945–954
Tripathy BC and Mohanty P (1986) Zinc inhibited electron transport of photosynthesis in isolated barley chloroplasts. Plant Physiol 66: 1174–1178
VanAssche F and Clijsters H (1986) Inhibition of photosynthesis in Phaseolus vulgaris by treatment with toxic concentration of zinc: Effects on electron transport and photophorylation. Physiol Plant 66: 717–721
Velthuys B (1983) Spectrophotometric methods of probing the donor side of Photosystem II. In: Inoue Y, Crofts A, Govindjee, Murata N, Renger G and Satoh K (eds) The Oxygen Evolving System of Photosynthesis, pp 83–90. Academic Press Japan, Inc., Tokyo
Witt AT (1975) Energy conservation in the functional membrane. In: Govindjee (ed) Bioenergetics of Photosynthesis, pp 493–554. Academic Press, New York
Woolhouse HW (1983) Toxicity and tolerance in the responses of plants to metals. In: Lange OL, Nobel PS, Osmond CB and Ziegler H (eds) Physiological Plant Ecology III. Encyclopedia of Plant Physiology, New Series, Vol 12C. Springer-Verlag, Berlin
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rashid, A., Bernier, M., Pazdernick, L. et al. Interaction of Zn2+ with the donor side of Photosystem II. Photosynth Res 30, 123–130 (1991). https://doi.org/10.1007/BF00042010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00042010