Abstract
Explants from leaves of in vitro-grown chrysanthemum (Dendranthema grandiflora Tzvel.) cultivars regenerated adventitious shoots without an intermediate callus phase. Puncturing explants with a brush increased regenerations, but in combination with cocultivation with Agrobacterium tumefaciens it had an adverse effect on shoot formation. The negative effect of brushing and cocultivation could be overcome by preculturing explants for 8 days. Preculture altered the location of transformed sites but did not inhibit transformation. Regeneration following cocultivation with Agrobacterium is also encouraged if alternative regeneration protocols are used that do not require brushing.
Similar content being viewed by others
Abbreviations
- BA:
-
benzyladenine
- GUS:
-
β-glucuronidase
- IAA:
-
indoleacetic acid
- NAA:
-
naphthaleneacetic acid
References
Bush SR, Earle ED & Langhans RW (1976) Plantlets from petal segments, petal epidermis and shoot tips of the periclinal chimera, Chrysanthemum morifolium ‘Indianapolis’. Amer. J. Bot. 63: 729–737
Colby SM, Juncosa AM & Meredith CP (1991) Cellular differences in Agrobacterium susceptibility and regenerative capacity restrict the development of transgenic grapevines. J. Amer. Soc. Hort. Sci. 116: 356–361
DeBlock M (1988) Genotype-independent leaf disc transformation of potato (Solanum tuberosum) using Agrobacterium tumefaciens. Theor. Appl. Genet. 76: 767–774
DeJong J & Van DeVrie M (1987) Components of resistance to Liriomyza trifolii in Chrysanthemum morifolium and Chrysanthemum pacificum. Euphytica 36: 719–724
Firoozabady E & Galbraith DW (1984) Presence of a plant cell wall is not required for transformation of Nicotiana by Agrobacterium tumefaciens. Plant Cell Tiss. Org. Cult. 3: 175–184
Fukai S & Oë M (1986) Effects of plant growth regulators on organ formation from leaf and stem segments of chrysanthemum (Dendranthema grandiflorum Kitamura) in vitro. Bull. Osaka Agr. Res. Cent. 23: 25–31
Fukai S, Chen Z & Oë M (1987) Cultivar differences in adventitious shoot formation from leaf segments of chrysanthemum (Dendranthema grandiflorum Ramat. Kitamura) Bull. Osaka Agr. Res. Cent. 24: 55–58
Hoekema A, Hirsch PR, Hooijkaas PJJ & Schilperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti plasmid. Nature 303: 179–180
Huitema JBM, Preil W, Gussenhoven GC & Schneidereit M (1989) Methods for the selection of low-temperature tolerant mutants of Chrysanthemum morifolium by using irradiated cell suspensions. I. Selection of regenerants in vivo under suboptimal temperature conditions. Plant Breeding 102: 140–147
Janssen BJ & Gardner RC (1989) Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol. Biol. 14: 61–72
Jefferson RA, Kavanagh TA & Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907
Kaul V, Miller RM, Hutchinson JF & Richards D (1990) Shoot regeneration from stem and leaf explants of Dendranthema grandiflora Tzelev (Syn. Chrysanthemum morifolium Ramat.) Plant Cell Tiss. Org. Cult. 21: 21–30
Koornneef M, Hanhart CJ & Martinelli L (1987) A genetic analysis of cell culture traits in tomato. Theor. Appl. Genet. 74: 633–641
Larkin PJ & Scowcroft WR (1981) Somaclonal variation — a novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 60: 197–214
Ledger SF, Deroles SC & Given NK (1991) Regeneration and Agrobacterium-mediated transformation of chrysanthemum. Plant Cell Rep. 10: 195–199
Lemieux CS, Firoozabady E & Robinson KEP (1990) Agrobacterium-mediated transformation of Chrysanthemum. In: DeJong J (Ed) Proc. Eucarpia Symposium on Integration of In Vitro Techniques in Ornamental Plant Breeding (pp 150–155). Pudoc, Wageningen, The Netherlands
Lu CY, Nugent G & Wardley T (1990) Efficient, direct plant regeneration from stem segments of chrysanthemum (Chrysanthemum morifolium Ramat. cv. Royal Purple) Plant Cell Rep. 8: 733–736
McHughen A, Jordan M & Feist G (1989) A preculture period prior to Agrobacterium inoculation increases the production of transgenic plants. J. Plant Physiol 135: 245–248
Miyazaki S, Kishida E, Tashiro Y & Kanazawa K (1979) Tissue culture of Chrysanthemum morifolium Ramat. V. Histological studies on the callus and shoot formation in stem segments cultured in vitro. Agric. Bull. Saga Univ. 46: 43–65
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Nadolska-Orczyk A & Malepszy S (1989) In vitro culture of Cucumis sativus L. 7. Genes controlling plant regeneration. Theor. Appl. Genet. 78: 836–840
Park YG & Son SH (1988) In vitro organogenesis and somatic embryogenesis from punctured leaf of Populus nigra x P. maximowiczii. Plant Cell Tiss. Org. Cult. 15: 95–105
Roest S & Bokelmann GS (1975) Vegetative propagation of Chrysanthemum morifolium Ram. in vitro. Scientia Hort. 3: 317–330
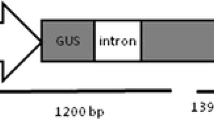
Vancanneyt G, Schmidt R, O'Connor-Sanchez A, Willmitzer L & Rocha-Sosa M (1990) Construction of an introncontaining marker gene: Splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol. Gen. Genet. 220: 245–259
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de Jong, J., Rademaker, W. & van Wordragen, M.F. Restoring adventitious shoot formation on chrysanthemum leaf explants following cocultivation with Agrobacterium tumefaciens . Plant Cell Tiss Organ Cult 32, 263–270 (1993). https://doi.org/10.1007/BF00042287
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00042287