Summary
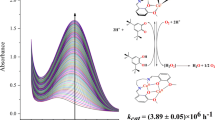
The kinetics and mechanism of ligand substitution reactions of tetraethylenepentamine nickel(II), Ni (Teren), and triethylenetetraamine nickel(II), Ni(Trien), with 4-(2-pyridylazo)resorcinol (parH2) have been studied spectrophotometrically at I=0.1 M (NaClO4) at 25°C. In both systems two distinct reaction steps are observed. The rapid first step follows the rate law d[Ni(Polyamine)(ParH2)]/dt=k1 [Ni(Polyamine)] [ParH2]. The formation of ternary complexes of Ni (Polyamine) with ParH2 has been investigated under second order equal concentration conditions. The values of second order rate constants for the Trien and Teren reactions are (2.1±0.2)×104 M−1s−1 and (7.8±0.6)×103 M−1s−1 respectively at pH=9.0, I=0.1 M and 25°C.
The rate law for the second step may be written as d[Ni(Par)2]/dt=k2[Ni(Polyamine)(ParH2)]. Values of k2 for the Trien and Teren systems are (2.5±0.1)×10−4 s−1 and (4.76±0.3)×10−5 s−1 respectively.
Similar content being viewed by others
References
A. K. S. Ahmed and R. G. Wilkins,J. Chem. Soc., 3700, (1959); 2895, 2901 (1960).
G. A. Melson and R. G. Wilkins,J. Chem. Soc., 2662 (1963).
D. W. Margerum, D. B. Rorabacher and J. F. G. Clark, Jr.,Inorg. Chem.,2, 667 (1963).
J. P. Jones and D. W. Margerum,J. Am. Chem. Soc.,92, 470 (1970) and references therein.
R. G. Wilkins,Acc. Chem. Res.,3, 408 (1970).
V. Stara and M. Kopanica,Collect. Czech. Chem. Commun.,37, 3545 (1972).
D. B. Rorabacher and D. W. Margerum,Inorg. Chem.,3, 382 (1964).
K. Kumar and P. C. Nigam,Inorg. Chem.,20, 1623 (1981) and refs therein.
H. C. Bajaj, K. Kumar and P. C. Nigam,Indian J. Chem.,20, 1070 (1981).
H. C. Bajaj and P. C. Nigam,Proceedings of the International Conference on Coordination Chemistry, Toulouse, France (1980), p. 487.
R. K. Steinhaus and J. A. Boersma,Inorg. Chem.,11, 505 (1972).
M. Tanaka, S. Funahashi, and K. Shirai,Anal. Chem. Acta,37, 437 (1967).
S. Funahashi and M. Tanaka,Inorg. Chem.,8, 2159 (1969).
D. D. Perrin and I. G. Sayce,Talanta,14, 833 (1967).
M. Eigen and R. G. Wilkins,Adv. Chem. Series,49, Chapter 3 (1965).
M. Eigen,Z. Elektrochem.,64, 115 (1960).
M. Eigen and K. Tamm,Z. Elektrochem.,66, 93 (1962).
S. Ooi, D. Carter and Q. Fernando,J. Chem. Soc. Chem. Commun., 1301 (1967).
R. M. Fuoss,J. Am. Chem. Soc.,80, 5059 (1958).
M. Eigen, W. Kruse, G. Maass and L. Demaeyer,Prog. React. Kinet.,2, 287 (1964).
M. Eigen,Z. Phys. Chem.,1, 176 (1954).
W. S. Melvin, D. P. Rablen, and G. Gordon,Inorg. Chem.,11, 488 (1972).
M. Cusumano,Inorg. Chim. Acta,25, 207 (1977).
D. P. Rablen, H. W. Dodgen and J. P. Hunt,Inorg. Chem.,15, 231 (1976).
K. Kustin, R. F. Pasternack and E. M. Weinstock,J. Am. Chem. Soc.,88, 4610 (1966).
A. Kowalak, K. Kustin, R. F. Pasternack and S. Petrucci,J. Am. Chem. Soc.,89, 3126 (1967).
T. Iwamato,Bull Chem. Soc. Jpn.,34, 605 (1961).
A. Corsin, I. Mai-Ling Yih, Q. Fernando and H. Freiser,Anal. Chem.,34, 1090 (1962).
B. Perlmutter-Hayman and R. Shinar,Inorg. Chem.,15, 2932 (1976);16, 385 (1977).
M. Grant, H. W. Dodgen and J. P. Hunt,J. Am. Chem. Soc.,22, 2321 (1970).
J. W. Neely and R. E. Connick,J. Am. Chem. Soc.,94, 8646 (1972).
D. N. Hague and K. Kinley,J. Chem. Soc., Dalton Trans., 249 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bajaj, H.C., Prasad, S., Naik, R.M. et al. Kinetics and mechanism of ligand substitution of mono(polyamine)-nickel(II) complexes with 4-(2-pyridylazo)resorcinol. Transition Met Chem 16, 511–517 (1991). https://doi.org/10.1007/BF01024320
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01024320