Abstract
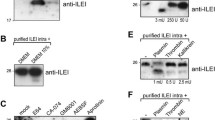
In the present paper, we have examined whether human tissue inhibitor of metalloprotease‐1 (hTIMP‐1) is able to exert a growth factor‐like effect on two clonal cell lines (BC‐3A and BC‐61), isolated from a parental line of human breast carcinoma cells (8701‐BC), and endowed with different growth and invasive behaviour ‘in vitro’ and in nude mouse. The data obtained indicate that only the more tumorigenic clonal cell line (BC‐61) is responsive to hTIMP‐1 treatment by increasing its proliferative rate in a dose‐dependent manner. It was also found that BC‐61 cells selectively express a transmembrane protein of about 80 kDa able to bind hTIMP‐1 ‘in vitro’ and ‘in vivo’ with high affinity (Kd of 0.07 ± 0.004 nM), and that treatment of BC‐61 cells with a proliferation‐promoting concentration of hTIMP‐1 is able to stimulate tyrosine‐targeted phosphorylation. The cumulative results obtained strongly support the hypothesis that hTIMP‐1, ‘classically’ regarded as a collagenase inhibitor, may be a crucial element of the extracellular signalling network during breast cancer development by controlling cell growth phenotype in autocrine and paracrine manner, and that intratumoural heterogeneity for the biological response to TIMP‐1 may exist within the composite cell population of the primary tumour site.
Similar content being viewed by others
References
Mignatti P, Rifkin DB: Biology and biochemistry of proteinases in tumour invasion. Physiol Rev 73: 161–185, 1993
Kleiner DE Jr, Stetler-Stevenson WG: Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol 5: 891–897, 1993
Baramova E, Foidar J-M: Matrix metalloproteinase family. Cell Biol Intl 19: 239–242, 1995
De Clerck YA: Tissue inhibitors of metalloproteinases: Comparative analysis and role in tumor progression. Cell Biol Intl 19: 244–245, 1995
Greene J, Wang M, Liu YE, Raymond LA, Rosen C, Shi YE: Molecular cloning and characterization of human tissue inhibitor of metalloproteinase 4. J Biol Chem 271: 30375–30380, 1996
Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM: Tissue inhibitor of metalloproteinases (TIMP, aka EPA): Structure, control of expression and biological functions. Pharmac Ther 59: 329–341, 1993
Howard EW, Bullen EC, Banda MJ: Preferential inhibition of 72 and 92 kDa gelatinases by tissue inhibitor of metalloproteinases-2. J Biol Chem 266: 13070–13075, 1991
Bian J, Wang Y, Smith MR, Kim H, Jacobs C, Jackman J, Kung H-F, Colburn NH, Sun Y: Suppression of in vivo tumour growth and induction of suspension cell death by tissue inhibitor of metalloproteinases (TIMP)-3. Carcinogenesis 17: 1805–1811, 1996
Schultz RM, Silberman S, Persky B, Bajkowski AS, Carmichael DF: Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res 48: 5539–5545, 1988
Yoshiji H, Gomez DE, Thorgeirsson UP: Enhanced RNA expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in human breast cancer. Int J Cancer 69: 131–134, 1996
Hansen Ree A, Flørenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad Ø: High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res 3: 1623–1628, 1997
Minafra S, Morello V, Glorioso F, La Fiura AM, Tomasino RM, Feo S, McIntosh D, Woolley DE: A new cell line (8701-BC) from primary ductal infiltrating carcinoma of human breast. Brit J Cancer 60: 185–192, 1989
Pucci-Minafra I, Minafra S, Alessandro R, Faccini AM: An ultrastructural evaluation of cell heterogeneity in invasive ductal carcinomas of the human breast II: An in vitro study. J Submicrosc Cytol Pathol 21: 488–499, 1989
Pucci-Minafra I, Minafra S, Faccini AM, Alessandro R: An ultrastructural evaluation of cell heterogeneity in invasive ductal carcinomas of the human breast I: An in vivo study. J Submicrosc Cytol Pathol 21: 475–488, 1989
Luparello C, Ginty AF, Gallagher JA, Pucci-Minafra I, Minafra S: Transforming growth factor-b1,-b2,-b3, urokinase and parathyroid hormone-related peptide expression in 8701-BC cells and clones. Differentiation 55: 73–80, 1993
Alessandro R, Minafra S, Pucci-Minafra I, Onisto M, Garbisa S, Melchiori A, Tetlow L, Woolley DE: Metalloproteinase and TIMP expression by the human breast carcinoma cell line 8701-BC. Int J Cancer 55: 250–255, 1993
Cifone MA, Fidler IH: Correlations of patterns of anchorageindependent growth with in vivo behaviour of cells from a murine fibrosarcoma. Proc Natl Acad Sci USA 77: 1039–1043, 1980
Ogata Y, Itoh Y, Nagase H: Steps involved in activation of the pro-matrix metalloproteinase 9 (progelatinase B)-tissue inhibitor of metalloproteinase-1 complex by 4-aminophenylmercuric acetate and proteinases. J Biol Chem 270: 18506–18111, 1995
Behrendt N, Rønne E, Plong M, Petri T, Løber D, Nielsen LS, Schleuning WD, Blasi F, Appella E, Danø K: The human receptor for urokinase plasminogen activator. J Biol Chem 265: 6453–6460, 1990
Minafra S, Giambelluca C, Andriolo M, Pucci-Minafra I: Cell–cell and cell–collagen interactions influence gelatinase production by human breast carcinoma cell line 8701-BC. Int J Cancer 62: 777–783, 1995
Barbieri R, Duro G, Costa MA, Izzo V: Simple and inexpensive dot-blot apparatus. Analyt Biochem 216: 461–462, 1994
Yamashita K, Suzuki M, Iwata H, Koike T, Hamaguchi M, Shinagawa A, Noguchi T, Hayakawa T: Tyrosine phosphorylation is crucial for growth signaling by tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2). FEBS Lett 396: 103–107, 1996
Shofuda K, Yasumitsu H, Nishihashi A, Miki K, Miyazaki K: Expression of three membrane-type matrix metalloproteinases (MT-MMPs) in rat vascular smooth muscle cells and characterization of MT3-MMPs with and without transmembrane domain. J Biol Chem 272: 9749–9754, 1997
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370: 61–65, 1994
Docherty AJP, Lyons A, Smith BJ, Wright EM, Stephens PE, Harris TJR, Murphy G, Reynolds JJ: Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-protentiating activity. Nature 318: 66–69, 1985
Avalos BR, Kaufman SE, Tomonaga M, Williams RE, Golde DW, Gasson JC: K562 cells produce and respond to human erythroid-potentiating activity. Blood 71: 1720–1725, 1988
Bertaux B, Hornebeck W, Eisen AZ, Dubertret L: Growth stimulation of human keratinocytes by tissue inhibitor of metalloproteinases. J Invest Dermatol 97: 679–685, 1991
Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K: Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. FEBS Lett 298: 29–32, 1992
Kikuchi K, Kadono T, Furue M, Tamaki K: Tissue inhibitor of metalloproteinase 1 (TIMP-1) may be an autocrine growth factor in scleroderma fibroblasts. J Invest Dermatol 108: 281–284, 1997
Heppner GH, Miller FR: The cellular basis of tumor progression. Int Rev Cytol 177: 1–56, 1998
Luparello C, Noël A, Pucci-Minafra I: Intratumoral heterogeneity for hsp90ß mRNA levels in a breast cancer cell line. DNA Cell Biol 16: 1231–1236, 1997
Luparello C, Burtis WJ, Raue F, Birch MA, Gallagher JA: Parathyroid hormone-related peptide and 8701-BC breast cancer cell growth and invasion in vitro: Evidence for growthinhibiting and invasion-promoting effects. Mol Cell Endocrinol 111: 225–232, 1995
Luparello C, Birch MA, Gallagher JA, Burtis WJ: Clonal heterogeneity of the growth and invasive response of a human breast carcinoma cell line to parathyroid hormone-related peptide fragments. Carcinogenesis 18: 23–29, 1997
Schillaci R, Luparello C, Minafra S: Type I and I-trimer collagens as substrates for breast carcinoma cells in culture. Effect on growth rate, morphological appearance and actin organization. Eur J Cell Biol 48: 135–141, 1989
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luparello, C., Avanzato, G., Carella, C. et al. Tissue inhibitor of metalloprotease (TIMP)‐1 and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res Treat 54, 235–244 (1999). https://doi.org/10.1023/A:1006121129382
Issue Date:
DOI: https://doi.org/10.1023/A:1006121129382