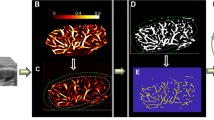
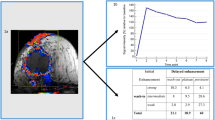
Abstract
Accurate predication of axillary node status by non-invasive diagnostic method would be of great value in cases of breast cancer. There have been few reports advocating digital subtraction angiography (DSA) as specifically advantageous for the detection of lymph node metastasis. IV (intravenous)-DSA was carried out on 42 patients with breast carcinoma using a DSA system with a matrix of 1024 × 1024×pixels. When a mass became stained in the axilla, it was considered to be metastatic. An immunohistochemical technique with JC70 antibody to platelet/endothelial cell adhesion molecules was used to evaluate the microvascular density (MVD) of the axillary lymph nodes. IV-DSA achieved a 76.2% sensitivity, 85.7% specificity, and 81.0% accuracy. The average MVD with JC70 antibody was 97.7 ± 44.4 in metastatic and 62.9 ± 23.6 in nonmetastatic nodes. MVD was significantly higher in the cancerous than in the noncancerous regions within lymph nodes. The MVD was 105 ± 38.4 in DSA-N(+) cases and was 57.8 ± 21.9 in DSA-N(−) cases, and the difference was statistically significant. In conclusion, IV-DSA is a useful diagnostic modality for detection of axillary lymph node metastasis. This new modality predicts lymph node status by assessing the neovascularization of the lymph node.
Similar content being viewed by others
References
Fisher B, Bauer M, Wickerham L, Redmond C, Fisher E: Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. Cancer 52: 1551–1557, 1983
Fisher ER: Pathologic findings from the National Surgical Adjuvant Project for breast cancers. Cancer 53: 712–723, 1984
Walsh R, Kornguth PJ, Soo MS, Bentley R, DeLong DM: Axillary lymph nodes: mammographic, pathologic, and clinical correlation. AJR 168(1): 33–38, 1997
Tohnosu N, Okuyama K, Koide Y, Kikuchi T, Awano T, Matsubara H, Sano T, Nakaichi H, Funami Y, Matsushita M: A comparison between ultrasonography and mammography, computed tomography and digital subtraction angiography for the detection of breast cancers. Surgery Today 23(8): 704–710, 1993
March DE, Wechsler RJ, Kurtz AB, Rosenberg AL, Needleman L: CT-pathologic correlation of axillary lymph nodes in breast carcinoma. J Computer Assisted Tomography 15(3): 440–444, 1991
Miyauchi M, Yamamoto N, Imanaka N, Matsumoto M: Computed tomography for preoperative evaluation of axillary nodal status in breast cancer. Breast Cancer 6(3): 243–248, 1999
de Freitas R Jr. Costa MV, Schneider SV, Nicolau MA, Marussi E: Accuracy of ultrasound and clinical examination in the diagnosis of axillary lymph nodes in breast cancer. Eur J Surg Oncol 17: 240–244, 1991
Mustonen P, Farin P, Kosunen O: Ultrasonographic detection of metastatic axillary lymph nodes in breast cancer. Ann Chir Gynaecol 79: 15–18, 1990
Tate JJ, Lewis V, Archer T, Guyer PG, Royle GT, Taylor I: Ultrasound detection of axillary lymph node metastasis breast cancer. Eur J Surg Oncol 15: 139–141, 1989
Mussurakis S, Buckley DL, Horsman A: Prediction of axillary lymph node status in invasive breast cancer with dynamic contrast-enhanced MR imaging. Radiology 203(2): 317–321, 1997
Yoshimura G, Sakurai T, Oura S, Suzuma T, Tamaki T, Umemura T, Kokawa Y, Yang Q: Evaluation of axillary lymph node status in breast cancer with MRI. Breast Cancer 6(3): 249–258, 1999
Watt AC, Ackerman LV, Shetty PC, Burke M, Flynn M, Grodsinsky C, Fine G: Differentiation between benign and malignant disease of the breast using digital subtraction angiography of the breast. Cancer 56(6): 1287–1292, 1985
Watanabe O, Haga S, Shimizu T, Imamura H, Kobayashi K, Kinoshita J, Kajiwara T, Fujibayashi M: Relationship between staining by digital subtraction angiography and vascularity in breast cancer: analysis of the time-density curve and the results of staining for factor VIII-related antigen/von Willebrand factor. Breast Cancer Res Treat 33(3): 269–273, 1995
Bjoerk L, Leven H: Intra-arterial DSA and duplex-sonography in detection of vascularized inguinal lymph node. Acta Radiol 31: 106–107, 1990
Weidner N, Semple JP, Welch WR, Folkman J: Tumor angiogenesis and metastasis - correlation in invasive breast carcinoma. New England J Medicine 324(1): 1–8, 1991
Fisher B, Redmond C, Fisher ER: the contribution of recent NSABP clinical trails of primary breast cancer therapy to an understanding of tumor biology - an overview of findings. Cancer 46(4 Suppl): 1009–1025, 1980
Dowlatshahi K, Fan M, Snider HC, Habib FA: Lymph node micrometastases from breast carcinoma: reviewing the dilemma. Cancer 80(7): 1188–1197, 1997
Lam WW, Yang WT, Chan YL, Stewart IE, Metreweli C, King W: Detection of axillary lymph node metastases in breast carcinoma by technetium - 99m sestamibi breast scintigraphy, ultrasound and conventional mammography. European J Nucl Med 23(5): 498–503, 1996
Folkman J: What is the evidence that tumors are angiogenesis dependent?. J Natl Cancer Inst 82(1): 4–6, 1990
Folkman J: Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med 1: 27–31, 1995
Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL: Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet 340(8828): 1120–1124, 1992
Feldman F, Habif D, Fleming R, Kanter I, Seaman W: Arteriography of the breast. Radiology 89: 1053–1061, 1967
Haga S, Watanabe O, Shimizu T, Imamura H, Kobayashi K, Kinoshita J, Nagumo H, Kajiwara T: Analysis of hte tumor staining obtained by preoperative IV-DSA for breast cancer patients: density and metastasis correlation. Breast Cancer Res Treat 43(2): 129–135, 1997
Furman-Haran E, Margalit R, Grobgeld D, Degani H: Dynamic contrast-enhanced magnetic resonance imaging reveals stress-induced angiogenesis in MCF7 human breast tumors. Proc Natl Acad Sci USA 93: 6247–6251, 1996
Liotta LA., Steeg PS, Stetler-Stevenson GS: Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell 64: 327–336, 1991
Herman PG, Kim C, de Sousa MAB, Mellins HZ: Microcirculation of the lymph node with metastasis. Am J Pathol 85: 333–348, 1976
Wu CH, Hsu MM, Chang YL, Hsieh FJ: Vascular pathology of malignant cervical lymphadenopathy: qualitative and quantitative assessment with power Doppler ultrasound. Cancer 83(6): 1189–1196, 1998
Parums DV, Cordell JL, Micklem K, Heryet AR, Gatter KC, Mason DY: JC70: a new monoclonal antibody that detects vascular endothelium associated on routinely processed tissue sections. J Clin Pathol 43: 752–757, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shimizu, T., Hino, K., Tauchi, K. et al. Predication of axillary lymph node metastasis by intravenous digital subtraction angiography in breast cancer, its correlation with microvascular density. Breast Cancer Res Treat 61, 261–269 (2000). https://doi.org/10.1023/A:1006449619475
Issue Date:
DOI: https://doi.org/10.1023/A:1006449619475