Summary
-
1.
The effects of long term administration of micromolar concentrations of the D2 antagonist haloperidol upon monoaminergic neurons in the snailLymnaea stagnalis was investigated.
-
2.
Treatment by bath application with 0.5–2.0 micromolar haloperidol, caused a significant, continuous depletion of dopamine levels in the nervous system as revealed by high performance liquid chromatography.
-
3.
A transient depletion of serotonin was also observed, but DOPA and norepinephrine levels were unaffected. Similar depletion of dopamine was observed after the land snail,Achatina fulica, was injected with haloperidol on each of 4 consecutive days.
-
4.
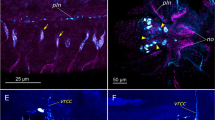
The depletion of dopamine as revealed with glyoxylate-induced fluorescence inLymnaea appears to be restricted to a subpopulation of catecholaminergic neurons which are immuno-negative for tyrosine hydroxylase, the synthetic enzyme responsible for the conversion of tyrosine to DOPA.
Similar content being viewed by others

References
Ashton, H. (1987).Brain Systems, Disorders, and Psychotropic Drugs, Oxford University Press, Oxford.
Baker, M. W., Vohra, M. M., and Croll, R. P. (1993). Serotonin depletors, 5,7-dihydroxytryptamine andp-chlorophenylalanine, cause sprouting in the CNS of the adult snail,Brain Res. 623:311–315.
Batten, T. F. C., Berry, P. A., Maqbool, A., Moons, L., and Vandesande, F. (1993). Immunolocalization of catecholamine enzymes, serotonin, dopamine and L-DOPA in the brain ofDicentrarachus labrax.Brain Res. Bull. 31:233–252.
Berry, M. S., and Cottrell, G. A. (1975). Dopamine: exitatory and inhibitory transmission from a giant dopamine neurone.Nature (London) New Biol. 242:250–253.
Carlberg, M., and Anctil, M. (1993). Biogenic amines in coelenterates.Comp. Biochem. Physiol. 106C:1–9.
Carlberg, M., Jegril, B., Lindbladh, C., and Rosengren, E. (1984). Enzymatic 5-hydroxylation of L-DOPA by a tyrosinase isolated from the sea anemoneMetridium senile.Gen. Pharmacol. 15:301–307.
Carlsson, A., and Lindqvist, M. (1963). Effect of chlorpromazine or haloperidol on formation of 3-emthoxytyramine and normetanephrine in mouse brain.Acta Pharmacol. Toxicol. 20:140–144.
Cournil, I., Helluy, S. M., and Beltz, B. (1994). Dopamine in the lobsterHomarus gammarus. I. Comparative analysis of dopamine and tyrosine hydroxylase immunoreactivities in the nervous system of the juvenile.J. Comp. Neurobiol. 344:455–469.
Croll, R. P., and Chiasson, B. J. (1989). Postembryonic development of serotoninlike immunoreactivity in the central nervous system of the snail.Lymnaea stagnalis.J. Comp. Neurol. 280:122–142.
Croll, R. P., and Chiasson, B. J. (1990). Distribution of catecholamines and of immunoreactivity to substances like vertebrate enzymes for the synthesis of catecholamines within the central nervous system of the snail,Lymnaea stagnalis.Brain Res. 525:101–114.
Ekström, P., Honkanen, T., and Borg, B. (1990). Development of tyrosine hydroxylase- and dopamine β-hydroxylase-immunoreactive neurons in a teleost, the three-spined stickleback.J. Chem. Neuroanat. 5:481–501.
Ekström, P., Honkanen, T., and Steinbusch, H. W. M. (1990). Distribution of dopamineimmunoreactive neuronal perikarya and fibres in the brain of teleost,Gasterosteus aculeatus.J. Chem. Neuroanat. 3:233–260.
Elekes, K., Kemenes, G., Hiripi, L., Geffard, M., and Benjamin, P. R. (1991). Dopamineimmunoreactive neurons in the central nervous system of the pond snailLymnaea stagnalis.J. Comp. Neurol. 307:214–224.
Gerschenfeld, H. M., and Pauparden-Tritsch, D. (1974). On the transmitter function of 5-hydroxytryptamine at excitatory and inhibitory monosynaptic junctions.J. Physiol. 243:457–481.
Goodman, L. S., Gilman, A., Rall, T. W., Nies, A. S., and Taylor, P. (1990).Goodman and Gilman's The Pharmacological Basis of Therapeutics, Pergamon Press, New York, pp. 386–404.
Grace, A. A. (1991). Phasic versus tonic dopamine release and the modulation of the dopamine system responsivity: A hypothesis for the etiology of schizophrenia.Neuroscience 41(1):1–24.
Heiss, W. D., Hoyer, J., and Thalhammer, G. (1976). Antipsychotic drugs and dopamine-mediated responses inAplysia neurones.J. Neural Transm. 39:187–208.
Hernadi, L., Juhos, S., and Elekes, K. (1993). Distribution of tyrosine-hydroxylase-immunoreactive and dopamine-immunoreactive neurons in the central nervous system of the snail,Helix pomatia.Cell Tissue Res. 274:503–513.
Juel, C. (1981). Preynaptic modulation of synaptic transmission inHelix pomatia: The effects of serotonin and dopamine antagonists.Neuropharm. 20:323–326.
Kabotyanski, E. A., and Sakharov, D. A. (1989). Catecholaminergic neurones in the pteropod molluscClione limacina.J. Evol. Biochem. Physiol. 25:198–207.
Kandel, E. R. (1991). Disorders in thought: Schizophrenia. InPrinciples of Neuroscience (E. R. Kandel, J. H. Schwartz, and T. M. Jessell, Eds.), Elsevier, New York, pp. 853–867.
Kemenes, G., Hiripi, L., and Benjamin, P. R. (1990). Behavioural and biochemical changes in the feeding system ofLymnaea induced by the dopamine and serotonin neurotoxins 6-hydroxydopamine and 5,6-dihydroxytryptamine.Phil. Trans. R. Soc. London. B 329:243–255.
Newlin, S. A., Schlapfer, W. T., and Barondes, S. H. (1980). Separate serotonin and dopamine receptors modulate the duration of post-tetanic potentiation at anAplysia synapse without affecting other aspects of synaptic transmission.Brain Res. 181:89–106.
Nilsson, J. L., and Carlsson, A. (1982). Dopamine-receptor agonists with apparent selectivity for autoreceptors: A new principle for antipsychotic action?Trends Pharm. Sci. 3:322–325.
Pani, A. K., and Anctil, M. (1994). Quantitative survey of biogenic monoamines, their precursors and metabolites in the coelenterateRenilla koellikeri.Biogenic Amines 10:161–180.
Peroutka, S. J., and Synder, S. H. (1980). Relationship of neuroleptic drug effects at brain dopamine, serotonin, α-adrenergic, and histamine receptors to clinical potency.Am. J. Psychiat. 137:1518–1522.
Sakharov, D., Voronezhskaya, E., and Nezlin, L. (1994). Chronic haloperidol: Neural correlates of motor disorders in an invertebrate model.Neuro Report 5:667–670.
Sloley, B. D., and Goldberg, J. I. (1991). Determination of ψ-glutamyl conjugates of monoamines by means of high-performance liquid chromatography within electrochemical detection and application to gastropod tissues.J. Chromatogr. 567:49–56.
Smeets, W. J. A. J., and Gonzalez, A. (1990). Are putative dopamine-accumulating cell bodies in the hypothalamic periventricular organ a primitive brain character of nonmammalian vertebrates?Neurosci. Lett. 114:248–252.
S.-Rozsa, K., and Perenyi, L. (1966). Chemical identification of the exitatory substance released in Helix heard during stimulation of the extracardial nerve.Comp. Biochem. Physiol. 19:105–113.
Torre, J. C. de la (1980). An improved approach to histofluorescence using the SPG method for tissue monoamines.J. Neurosci. Methods 3:1–5.
Voronezhskaya, E. E., Pavlova, G. A., and Sakharov, D. A. (1993). Effects of haloperidol and methylergometrine on embryonic motility and development of the pond snailLymnaea stagnalis.Ontogenez 24:40–47 (in Russian).
Walker, R. J. (1986). Neurobiology and behaviour. InThe Mollusca, Vol. 9, Part 2 (A. O. D. Willows, Ed.), Academic Press, Orlando, FL, pp. 279–485.
Wolf, M. A., and Roth, R. H. (1990). Autoreceptor regulation of dopamine synthesis.Ann. N. Y. Acad. Sci. 604:323–343.
Zetterström, T., Sharp, T., and Ungerstedt, U. (1985). Effect of neuroleptic drugs on striatal dopamine release and metabolism in the awake rat studied byintracerebral dialysis.Eur. J. Pharmacol. 106:27–37.
Zigmond, R. E., Schwartzchild, M. A., and Rittenhouse, A. R. (1988). Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation.Annu. Rev. Neurosci. 12:415–461.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakharov, D.A., Voronezhskaya, E.E., Nezlin, L. et al. Tyrosine hydroxylase-negative, dopaminergic neurons are targets for transmitter-depleting action of haloperidol in the snail brain. Cell Mol Neurobiol 16, 451–461 (1996). https://doi.org/10.1007/BF02150226
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02150226