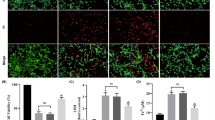
Abstract
1. Hippocampal CA1 neurons are the most vulnerable to transient cerebral ischemia. However, the mechanism has not been fully understood.
2. The mRNAs for 72-kd (HSP72) and 73-kd (HSC73) heat shock proteins (HSPs), which are located mainly in the cytoplasm, were greatly induced together in CA1 cells, with a peak at 1–2 days in gerbils. However, immunoreactive HSP72 protein was only minimally expressed in CA1 neurons.
3. The mRNA for mitochondrial HSP60 began to increase at 3 hr in CA1 cells and was sustained until 1 day.
4. The level of mRNA for cytochrome c oxidase subunit I (COX-I) progressively decreased in CA1 neurons after a transient ischemia and completely disappeared at 7 days. The activity of cytochrome c oxidase (COX) protein also showed an early decrease in CA1 cells and was followed by a reduction in the level of COX-I DNA after 2 days.
5. These results suggest that HSP gene inductions were inhibited at the translational level but that mitochondrial DNA expression was disturbed at the transcriptional level. A disturbance of mitochondrial DNA expression could cause progressive failure of energy production of CA1 cells that eventually results in neuronal cell death.
Similar content being viewed by others
REFERENCES
Abe, K., Tanzi, R. E., and Kogure, K. (1991). Induction of HSP70 mRNA after transient ischemia in gerbil brain. Neurosci. Lett. 125:166–168.
Abe, K., Sato, S., Kawagoe, J., Lee, T.-H., and Kogure, K. (1993a). Isolation and expression of an ischemia-induced gene from gerbil cerebral cortex by subtractive hybridization. Neurol. Res. 15:23–28.
Abe, K., Kawagoe, J., Lee, T.-H., Aoki, M., and Kogure, K. (1993b). Change of mitochondrial DNA and heat shock gene expressions in gerbil hippocampus after transient brain ischemia. J. Cereb. Blood Flow Metab. 13:773–780.
Abe, K., Aoki, M., Kawagoe, J., Yoshida, T., Hattori, A., Kogure, K., and Itoyama, Y. (1995). Ischemic delayed neuronal death: A mitochondrial hypothesis. Stroke 26:1478–1489.
Aoki, M., Abe, K., Kawagoe, J., and Kogure, K. (1993). Acceleration of HSP70 and HSC70 gene expression in preconditioned gerbil hippocampus. J. Cereb. Blood Flow Metabol. 13:781–788.
Aoki, M., Abe, K., Yoshida, T., Hattori, A., Kogure, K., and Itoyama, Y. (1995). Early immunohistochemical changes of microtubule based motor proteins in gerbil hippocampus after transient ischemia. Brain Res. 669:189–196.
Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K., and Watson, J. D. (1989). Molecular Biology of the Cell., 2nd ed., Garland, New York, pp. 389–401.
Arai, H., Lust, W. D., and Passonneau, J. V. (1982). Delayed metabolic changes induced by 5 min of ischemia in gerbil brain. Trans. Am. Soc. Neurochem. 13:s177.
Arai, H., Passonneau, J. V., and Lust, W. D. (1986). Energy metabolism in delayed neuronal death of CA1 neurons of the hippocampus following transient ischemia in the gerbil. Metab. Brain Dis. 1:263–278.
Benveniste, H., Drejer, J., Schousboe, A., and Diemer, N. H. (1984). Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 43:1369–1374.
Bodsch, W., Takahashi, K., Barbier, B., Ophoff, G., and Hossmann, K. A. (1985). Cerebral proteins and ischemia. Prog. Brain Res. 63:197–210.
Deshpande, J., Bergstedt, K., Linden, T., Kalimo, H., and Wieloch, T. (1992). Ultrastructural changes in the hippocampal CA1 region following transient cerebral ischemia: Evidence against programmed cell death. Exp. Brain Res. 88:91–105.
Gething, M. J., and Sambrook, J. (1992). Protein folding in the cell. Nature 355:33–45.
Hevner, R. F., and Wong-Riley, M. T. T. (1991). Neuronal expression of nuclear and mitochondrial genes for cytochrome oxidase (CO) subunits analysed by in situ hybridization: Comparison with CO activity and protein. J. Neurosci. 11:1942–1958.
Hickey, E., Brandon, S. E., Sadis, S., Smale, G., and Weber, L.A. (1986). Molecular cloning of sequences encoding the human heat shock proteins and their expression during hyperthermia. Gene 43:147–154.
Hightower, L. E. (1991). Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66:191–197.
Ikebe, S., Tanaka, M., Ohno, K., Sato, W., Hattori, K., Kondo, T., Mizuno, Y., and Ozawa, T. (1990). Increase of deleted mitochondrial DNA in the striatum in Parkinson's disease and senescence. Biochem. Biophys. Res. Commun. 170:1044–1048.
Imdall, A., and Hossmann, K. A. (1986). Morphometric evaluation of postischemic capillary perfusion in selectively vulnerable areas of gerbil brain. Acta Neuropathol. (Berlin) 69:267–271.
Izumiyama, K., Kogure, K., Kataoka, S., and Nagata, T. (1987). Quantitative analysis of glucose after transient ischemia in the gerbil hippocampus by light and electron microscope radioautography. Brain Res. 416:175–179.
Kawagoe, J., Abe, K., Sato, S., Nagano, I., Nakamura, S., and Kogure, K. (1992). Distributions of heat shock protein-70 mRNA and heat shock cognate-70 mRNA after transient global ischemia in gerbil brain. J. Cereb. Blood Flow. Metab. 12:794–801.
Kirino, T. (1982). Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 239:57–69.
Maniatis, T., Fritsch, E. F., and Sambrook, J. (1982). Molecular Cloning, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, pp. 187–206.
Mita, S., Schmit, B., Schon, E. A., DiMauro, S., and Bonilla, E. (1989). Detection of “deleted” mitochondrial genomes in cytochrome C oxidase-deficient muscle fibers of a patient with Keans-Sayre syndrome. Proc. Natl. Acad. Sci. USA 86:9509–9513.
Nakata, N., Kato, H., Liu, Y., and Kogure, K. (1992). Effects of pretreatment with sublethal ischemia on the extracellular glutamate concentrations during secondary ischemia in the gerbil hippocampus evaluated with intracerebral microdialysis. Neurosci. Lett. 138:86–88.
Ozawa, K., Seta, K., Araki, H., and Handa, H. (1967). The effect of ischemia on mitochondrial metabolism. J. Biochem. 61:512–514.
Parker, W. D., Jr., Boyson, S. J., Ludder, A. S., and Parks, J. K. (1990). Evidence for a defect in NADH:ubiquitin oxidoreductase (Complex I) in Huntington's disease. Neurology 40:1231–1234.
Pulsinelli, W. A., and Duffy, T. E. (1983). Regional energy balance in rat brain after transient forebrain ischemia. J. Neurochem. 40:1500–1503.
Pulsinelli, W. A., Brieley, L. B., and Plum, F. C. (1982). Temporal profile of neuronal damage in a model of transient ischemia. Ann. Neurol. 11:491–498.
Rothman, S. M., and Olney, J. W. (1986). Glutamate and pathophysiology of hypoxic brain damage. Ann. Neurol. 19:105–111.
Sato, S., Abe, K., Kawagoe, J., Aoki, M., and Kogure, K. (1992). Isolation of complementary DNAs for HSP70 and HSC70 genes and the expressions in postischaemic gerbil brain. Neurol. Res. 14:375–380.
Schapira, A. H., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P., and Marsden, C. D. (1990). Mitochondrial complex I deficiency in Parkinson's disease. J. Neurochem. 54:823–827.
Schoffner, J. M., Walts, R. L., Juncos, J. L., Torrini, A., and Wallace, D. C. (1991). Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann. Neurol. 30:332–339.
Seligman, A. M., Karnovsky, M. J., Wasserkrug, H. L., and Hanker, J. S. (1968). Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J. Cell Biol. 38:1–14.
Vass, K., Welch, W. J., and Nowak, T.S., Jr. (1988). Localization of 70-kDa stress protein induction in gerbil brain after ischemia. Acta Neuropathol. 77:128–135.
Wagner, K. R., Kleinholz, M., and Myers, R. E. (1990). Delayed decreases in specific brain mitochondrial electron transfer complex activities and cytochrome concentrations following anoxia/ischemia. J. Neurol. Sci. 100:142–151.
Wallace, D. C., Zheng, X., Lott, M. T., Shoffer, J. M., Hodge, J. A., Kelly, R. I., Epstein, C. M., and Hopkins, L. C. (1988). Familial mitochondrial encephalopathy (MERRF): Genetic, pathophysiologic, and biochemical characterization of a mitochondrial DNA disease. Cell 55:601–610.
Westerberg, E., Deshpande, J. K., and Wieloch, T. (1987). Regional differences in arachidonic release in rat hippocampal CA1 and CA3 regions during cerebral ischemia. J. Cereb. Blood Flow Metab. 7:189–192.
Wieloch, T. (1985). Neurochemical correlates to selective neuronal vulnerability. Prog. Brain Res. 63:69–85.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abe, K., Kawagoe, J., Aoki, M. et al. Stress Protein Inductions After Brain Ischemia. Cell Mol Neurobiol 18, 709–719 (1998). https://doi.org/10.1023/A:1020694205003
Issue Date:
DOI: https://doi.org/10.1023/A:1020694205003