Abstract
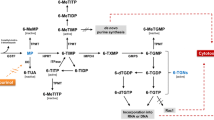
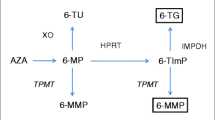
Azathioprine (AZA) is used in the treatment of patients with refractory inflammatory bowel disease; however, its use is limited because of systemic toxicity associated with long-term use. Ileocecal delivery of AZA might be advantageous if local intestinal therapeutic effects could be provided with decreased systemic side effects. Decreased cecal systemic absorption would allow higher dosages of AZA to be administered. A two-phase study was performed to compare the systemic exposure of AZA and 6-mercaptopurine (6-MP) following administration of AZA into the stomach, jejunum, and cecum and to compare the systemic exposure to AZA and 6-MP following administration of three different dosages of AZA into the cecum. In phase I, six healthy male volunteers received three 50 mg sequential doses of AZA via an oral tube directly placed into the stomach, jejunum, and cecum, respectively. In phase II, six healthy male volunteers received three different dosages (50, 300, 600 mg of AZA) into the cecum. Plasma concentrations of AZA and 6-MP at various times were quantified and area under the plasma concentration-time curve (AUC) and mean residence time (MRT) were determined. No significant differences in the AUC of AZA were seen at the different sites. The AUC of 6-MP following administration of AZA into the jejunum (67.0 ± 30.1 ng×hr/ml) was higher compared to the stomach (39.9 ± 38.1 ng/hr/ml) and cecum (29.2 ± 10.9 ng×hr/ml). Jejunal absorption was 68% higher than absorption from the stomach and 129% higher than that of the cecum. Gastric absorption was 27% higher than that of the cecum. Increased dosages given into the cecum resulted in increased AUCs of AZA and 6-MP. The AUCs of AZA following 50, 300, and 600 mg dosages were 16.9 ± 7.4, 52.3 ± 67.2, and 132 ± 151 ng×hr/ml, respectively, and the AUCs of 6-MP were 22.2 ± 14.9, 63.4 ± 50.6, and 104 ± 115 ng×hr/ml, respectively. Systemic exposure to 6-MP is reduced following administration of AZA into the cecum, most likely secondary to reduced absorption of 6-MP from the colon. Higher dosages of AZA presented to the cecum do result in increased systemic absorption, but may still allow more drug to be administered with less toxicity than the same dose received orally.
Similar content being viewed by others
REFERENCES
Sandborn WJ: A review of immune modifier therapy for inflammatory bowel disease: Azathioprine, 6–mercaptopurine, cyclosporine, and methotrexate. Am J Gastroenterol 91:423–433, 1996
O'Donoghue DP, Dawson AW, Powell-Tuck J, Bown RL, Lennard-Jones JE: Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn' disease. Lancet 2:955–957, 1978
Ewe K, Press AG, Singe CC, Stufler M, Ueberschaer B, Hommel G, Buschenfelde K: Azathioprine combined with prednisone or monotherapy with prednisone in active Crohn' disease. Gastroenterology 105:367–372, 1993
Pearson DC, May GR, Fick GH, Sutherland LR: Azathioprine and 6–mercaptopurine in Crohn' disease: A meta-analysis. Ann Intern Med 122:132–142, 1995
Jewell DP, Truelove SC: Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. BMJ 4:627–630, 1974
Kirk AP, Lennard-Jones JE: Controlled trial of azathioprine in chronic ulcerative colitis. BMJ 284:1291–1292, 1982
Hawthorne AB, Logan RFA, Hawkey CJ, Foster PN, Axon ATR, Swarbrick ET, Scott BB, Lennard-Jones JE: Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 305:20–22, 1992
Rosenberg JL, Wall AJ, Levin B, Binder HJ, Birsner JB: A controlled trial of azathioprine in the management of chronic ulcerative colitis. Gastroenterology 69:96–99, 1975
O'Donoghue DP, Dawson AM, Powell-Tuck J: Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn' disease. Lancet 2:955–957, 1978
Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI: Six-mercaptopurine in the management of inflammatory bowel disease: Short-and long-term toxicity. Ann Intern Med 111:641–649, 1989
Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE: Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 34:1081–1085, 1995
Lennard L, Rees CA, Lilleyman JS, Maddocks JL: Childhood leukemia: A relationship between intracellular 6–mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol 16:359–363, 1983
Posthuma EFM, Westendorp RGJ, van der Sluys Veer A, Kluin-Nelemans JC, Kluin PM, Lamers CBHW: Faithful infectious mononucleosis: A severe complication in the treatment of Crohn' disease with azathioprine. Gut 36:311–313, 1995
Schroeder KW, Tremaine WJ, Ilstrup DM: Coated oral 5–aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med 317:1625–1629, 1987
Edsbacker S, Wollmer P, Nilsson A, Nilsson M: Pharmacokinetics and gastrointestinal transit of budesonide controlled ileal release (CIR) capsules. Gastroenterology 104:A695, 1993
Greenberg GR, Feagan BG, Martin F, Sutherland L, Thomson ABR, Williams CN, Nillson LG, Persson T, Canadian Inflammatory Bowel Disease Study Group: Oral budesonide for active Crohn' disease. N Engl J Med 331:836–841, 1994
Van Os EC, Zins BJ, Sandborn WJ, Mays DC, Tremaine WJ, Mahoney DW, Zinsmeister AR, Lipsky JJ: Azathioprine pharmacokinetics after intravenous, oral, delayed release oral and rectal foam administration. Gut 39:63–68, 1996
Odlind B, Hartvig P, Linstrom B, Lonnerholm GL, Tufveson G, Grefberg N: Serum azathioprine and 6–mercaptopurine levels in immunosuppressive activity after azathioprine in uremic patients. Int J Immunopharmacol 8:1–11, 1986
Zimm S, Collins JM, Riccardi R, O'Neil D, Narang PK, Chabner B, Poplack DG: Variable bioavailability of oral mercaptopurine. Is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med 308:1005–1009, 1983
Weinshilboum RN, Sladek SL: Mercaptopurine pharmacogenetics: Monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 32:651–652, 1980
Lennard L, Van Loon JA, Lilleyman JS, Weinshilboum RM: Thiopurine pharmacogenetics in leukemia: Correlation of erythrocyte thiopurine methyltransferase activity and 6–thioguanine nucleotide concentrations. Clin Pharmacol Ther 41:18–25, 1987
Lennard L, Van Loon JA, Weinshilboum RM: Pharmacogenetics of acute azathioprine toxicity: Relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther 46:149–154, 1989
Williams MF, Dukes GE, Heizer W, Han YH, Hermann DJ, Lampkin T, Hak LJ: Influence of gastrointestinal site of drug delivery on the absorption characteristics of ranitidine. Pharm Res 9:1190–1194, 1992
Elion GB: The comparative metabolism of Imuran and 6–mercaptopurine in man. Proc Am Assoc Cancer Res 10:21, 1969
De Miranda P, Beacham LM, Creagh TH: The metabolic fate of the methylnitroimidazole moiety of azathioprine in the rat. J Pharmacol Exp Ther 187:588–601, 1973
Lennard L: The clinical pharmacology of 6–mercaptopurine. Eur J Clin Pharmacol 43:329–339, 1992
El-Yazigi A, Wahab FA: Pharmacokinetics of azathioprine after repeated oral and single intravenous administration. J Clin Pharmacol 33:522–526, 1993
Zins BJ, Sandborn WJ, McKinney JA, Mays DC, Van Os EC, Tremaine WJ, Mahoney DW, Zinsmeister AR, Lipsky JJ: A dose-ranging study of azathioprine pharmacokinetics after single-dose administration of a delayed-release oral formulation. J Clin Pharmacol 37:38–46, 1997
Kurowski V, Iven H: Plasma concentrations and organ distribution of thiopurines after oral application of azathioprine in mice. Cancer Chemother Pharmacol 28:7–14, 1991
Erdmann GR, France LA, Bostrom BC, Canafax DM: A reversed-phase high-performance liquid chromatography approach in determining total red blood cell concentrations of 6–thioguanine, 6–mercaptopurine, methylthioguanine, and methylmercaptopurine in a subject receiving thiopurine therapy. Biomed Chromat 4:47–51, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gervasio, J.M., Brown, R.O., Lima, J. et al. Sequential Group Trial to Determine Gastrointestinal Site of Absorption and Systemic Exposure of Azathioprine. Dig Dis Sci 45, 1601–1607 (2000). https://doi.org/10.1023/A:1005573229786
Issue Date:
DOI: https://doi.org/10.1023/A:1005573229786