Summary
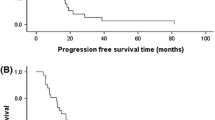
The Southwest Oncology Group studied the response rate and toxicity of mitoxantrone (7.5 or 10 mg/m2 to 12.0 mg/m2) and cis-platinum (100 mg/m2) in 30 patients with advanced breast cancer as second-line therapy. There were 2 partial responses in 29 eligible patients. Toxicity was considerable, with 27 patients having grade 3 or 4 toxicity. Grade 3–4 toxicity included vomiting, thrombocytopenia, granulocytopenia, leukopenia and anemia. The combination of mitoxantrone plus cis-platinum has minimal activity as second-line therapy in metastatic breast cancer.
Similar content being viewed by others
References
Schabel FM, Corbett TH, Griswold DP, Laster WR, Trafer MW: Therapeutic activity of mitoxantrone and ametantrone against murine tumors. Cancer Treatment Reviews 10:13–21, 1983 (Suppl)
Mouridsen HT, Rose C, Nooy MA, van Oosterom AT: Mitoxantrone as first line cytotoxic therapy in advanced breast cancer: preliminary results of a phase II study. Cancer Treatment Reviews 10:47–52, 1983 (Suppl)
Smith IE, Stuart-Harris N, Paulidis N, Bozek T: Mitoxantrone (Novantrone) as single agent and in combination chemotherapy in the treatment of advanced breast cancer. Cancer Treatment Reviews 10:37–40, 1983 (Suppl)
Neidhart JA, Gochnour D, Roach RW, Steinberg JA, Young D: Mitoxantrone versus doxorubicin in advanced breast cancer: a randomized cross-over trial. Cancer Treatment Reviews 10:41–46, 1983 (Suppl)
Crossley RJ: Clinical safety and tolerance of mitoxantrone (Novantrone). Cancer Treatment Reviews 10:29–36, 1983 (Suppl)
Cowan JD, Neidhart J, McClure S, Coltman CA Jr, Gumbart C, Martino S, Hutchins LF, Stephens RL, Vaughan CB, Osborne CK: Randomized trial of doxorubicin, bisantrene, and mitoxantrone in advanced breast cancer: A Southwest Oncology Group study. J Natl Cancer Inst 83:1077–1084, 1991
Forastiere HA, Hakes TB, Wittes JT, Wittes AE: Cisplatin in the treatment of metastatic breast carcinoma. Am J Clin Oncol 5:243–247, 1983
Sledge GW, Roth BJ: Cisplatin in the management of breast cancer. Sem in Oncol 16:110–115, 1989 (Suppl)
Kolaric K, Roth A; Phase II clinical trial of cis-dichlordiammine platinum (cis-DDP) for autotumorigenic activity in previously untreated patients with metastatic breast cancer. Cancer Chemother Pharmacol 11:108–112, 1983
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atiba, J.O., Green, S.J., Hynes, H.E. et al. Phase II evaluation of mitoxantrone plus cis-platinum in patients with advanced breast cancer. Invest New Drugs 12, 129–132 (1994). https://doi.org/10.1007/BF00874442
Issue Date:
DOI: https://doi.org/10.1007/BF00874442