Abstract
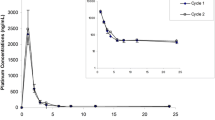
Ormaplatin (NSC 363812, tetraplatin) is a stable platinum (IV) analog which has exhibited activity against cisplatin-resistant cell lines. A phase I trial of ormaplatin administered as a 1-h infusion every 4 weeks was performed. Forty-one patients received 101 cycles of drug over the dose range 4–128 mg/m2. The dose-limiting toxicity was reversible thrombocytopenia and granulocytopenia. Minimal myelosuppression was observed at dose levels ≤ 78 mg/m2, while grade 3 or 4 myelosuppression (thrombocytopenia and/or granulocytopenia) was seen in 4/8 patients at 98 mg/m2 and 4/5 patients at 123 mg/m2. Nausea and vomiting was observed at all dose levels but was controlled with antiemetic premedication. Neurotoxicity was observed in 5/41 patients and the incidence appeared related to cumulative dose rather than to dose level or drug clearance. Platinum was measured by furnace atomic absorption spectrophotometry. Ormaplatin-derived plasma ultrafilterable platinum (UF-Pt) exhibited linear pharmacokinetics over the dose range studied. The mean total body clearance of UF-Pt was 135 ml/min/m2 and the mean elimination half-life (t1/2β) was 13.6 h. Ormaplatin exhibited a high degree of protein binding, with more than 70% of platinum protein bound by the end of the infusion. Urinary excretion of platinum accounted for 37% of the total dose of ormaplatin in 24 hours. A phase II dose of 98 mg/m2 is recommended for testing in a patient population with cisplatin-refractory disease.
Similar content being viewed by others
References
Reed E, Kohn KW: Platinum analogues. In: Chabner BA, Collins JM (eds) Cancer Chemotherapy Principles and Practice. JB Lippincott Co, Philadelphia, 1990, pp 465–490
Loehrer PJ, Einhorn LH: Drugs five years later: Cisplatin. Ann Intern Med 100: 704–713, 1984
Christian MC: The current status of new platinum analogs. Seminars in Oncol 19: 720–733, 1992
Burchenal JH, Irani G, Kern K, Lokys L, Turkevich J: 1-2 diaminocyclohexane platinum derivatives of potential clinical value. Cancer Res 74: 146–155, 1980
Eastman A: Glutathione-mediated activation of anticancer platinum (IV) complexes. Biochem Pharmacol 36: 4177–4178, 1987
Anderson WK, Quafliato DA, Haugwitz RD, Narayanan VL, Wolpert-DeFilippes MK: Synthesis, physical properties and antitumor activity of tetraplatin and related tetrachloroplatinum (IV) stereoisomers of 1,2-diaminocyclohexane. Cancer Treat Rep 70: 997–1002, 1986
Gibbons GR, Wyrick S, Chaney SG: Rapid reduction of tetrachloro (DL-trans)-1,2-diaminocyclohexaneplatinum (IV) (tetraplatin) in RPMI 1640 tissue culture medium. Cancer Res 49: 1402–1407, 1989
Rahman A, Roh JK, Wolpert-DeFilippes MK, Goldin A, Venditti JM, Woolley PV: Therapeutic and pharmacological studies of tetrachloro(d,1-trans)1,2-diaminocyclohexane platinum (IV) (tetraplatin), a new platinum analogue. Cancer Res 48: 1745–1752, 1988
Smith JH, Smith MA, Litterst CL, Copley MP, Uozumi J, Boyd MR: Comparative toxicity and renal distribution of the platinum analogs tetraplatin, CHIP and cisplatin at equimolar doses in the Fisher 344-rat. Fund Appl Toxicol 10: 45–61, 1988
Smith MA, Smith JH, Litterst CL, Copley MP, Uozumi J, Boyd MR: In vivo biochemical indices of nephrotoxicity of the platinum analogs tetraplatin, CHIP and cisplatin in the Fisher 344 rat. Fund Appl Toxicol 10: 62–72, 1988
McPherson RA, Roh JK, Komanduri K, Mhatre R, Wooley PV, Rahman A: Toxicological evaluation of tetraplatin (NSC 363812) in comparison to CDDP. Proc Am Assoc Cancer Res 27: 291, 1986
Kris MG, Gralla RJ, Tyson LB, Clark RA, Kelsen DP, Reilly LK, Groshen S, Bosl GJ, Kalman LA: Improved control of cisplatin-induced emesis with high-dose metoclopramide and with combinations of metoclopramide, dexamthasone, and diphenhydramine. Cancer 55: 527–534, 1985
Robins HI, Cohen JD, Schmitt CL, Tutsch KD, Feierabend C, Arzoomanian RZ, Alberti D, d'Oleire F, Longo W, Heiss C, Rushing D, Love R, Spriggs D: Phase I clinical trial of carboplatin and 41.8 _C whole-body hyperthermia in cancer patients. J Clin Oncol 111: 1787–1794, 1993
Akaike H: An information criterion (AIC). Math Sci 14: 5–9, 1976
Gibaldi M, Perrier D: Pharmacokinetics, 2nd Edition. Marcel Dekker Inc, New York, 1982, pp 45–111
Egorin MJ, VanEcho DA, Tipping SJ, Olman EA, Whitacre MY, Thompson BW, Aisner J: Pharmacokinetics and dosage reduction of cis-diammine (1,1cyclobutanedicarboxylato) platinum in patients with impaired renal function. Cancer Res 44: 5432–5438, 1984
Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E: Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: 1748–1756, 1989
Schilder RJ, LaCreta FP, Perez RP, Johnson SW, Brennan JM, Rogatko A, Nash S, McAleer C, Hamilton TC, Roby D, Young RC, Ozols RF, O'Dwyer PJ: Phase I and pharmacokinetic study of ormaplatin (tetraplatin, NSC 363812) administered on a day 1 and day 8 schedule. Cancer Res 54: 709–717, 1994
O'Rourke TJ, Weiss GR, New P, Burris HA, Rodriguez G, Eckhardt J, Hardy J, Kuhn JG, Fields S, Clark GM, Von Hoff DD: Phase I clinical trial of ormaplatin (tetraplatin, NSC 363812). Anti-Cancer Drugs 5: 520–526, 1994
Figg WD, Christian MC, Lush R, Link CJ, Davis P, Kohn E, Sarosy G, Rothenberg ML, Weiss RB, Ryan N, Jacobs J, Reed E: Pharmacokinetics of elemental platinum (ultrafiltrate and total) after a thirty-minute intravenous infusion of ormaplatin. Biopharm Drug Disp 18: 347–359, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tutsch, K.D., Arzoomanian, R.Z., Alberti, D. et al. Phase I clinical and pharmacokinetic study of an one-hour infusion of ormaplatin (NSC 363812). Invest New Drugs 17, 63–72 (1999). https://doi.org/10.1023/A:1006223100561
Issue Date:
DOI: https://doi.org/10.1023/A:1006223100561