Summary
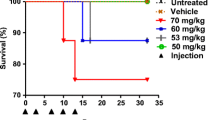
The LD 50 of Z 7557 in mice was between 500 and 625 mg/kg after i.v. and around 2310 mg/kg after oral administration. The corresponding LD 10 was around 435 mg/kg (i.v.) or 1100–1250 mg/kg (p.o.), respectively.
The LD 50 values in rats were in the range of 250–310 mg/kg after i.v. administration and around 1000–1250 mg/kg if given orally.
With repeated daily i.v. injections only 70% of the daily dose contributed to lethality. A second administration of Z 7557 to rats after reversal of all toxic signs from the first administration induced the same symptoms and degree of toxicity as the initial injection.
Reversible myelosuppression was the predominant feature of toxicity in mice and rats, but at equimolar doses this myelotoxicity was less than half that of cyclophosphamide (CP). First signs of immunosuppression were seen only at 100 mg/kg i.v.
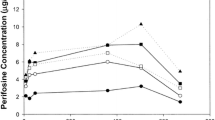
No severe urotoxicity was observed in rats with single i.v. doses up to 192 mg/kg. This might be due to the fast and complete renal excretion of the thiol moiety of Z 7557, whereas the activated oxazaphosphorine component occurred in the urine only to a much smaller amount, as could be shown by a pilot pharmacokinetic study.
In conclusion, Z 7557 appeared to have an overall tolerance in rats and mice similar to cyclophosphamide but was clearly less toxic with respect to the bone marrow, the immune system and the urinary tract.
Similar content being viewed by others
References
Brock N: Comparative pharmacologic study in vitro and in vivo with cyclophosphamide (NSC-26271), cyclophosphamide metabolites, and plain nitrogen mustard compounds. Cancer Treat Rep 60:301–307, 1976
Kirschstein RL: Editorial: Cyclamates. Hum Pathol 5:498–499, 1974
Brock N, Pohl J, Stekar J, Scheef W: Studies on the urotoxicity of oxazaphosphorine cytostatics and its prevention — III. Profile of action of sodium 2-mercaptoethane sulfonate (mesna). Eur J Cancer Clin Oncol 18:1377–1387, 1982
Brock N, Schneider B: Models and methods for the assessment of cumulation of drug effects. Arzneim-Forsch Drug Res 30:1034–1040, 1980
Brock N, Pohl J, Stekar J: Studies on the urotoxicity of oxazaphosphorine cytostatics and its prevention — I. Experimental studies on the urotoxicity of alkylating compounds. Eur J Cancer 17:595–607, 1981
Brock N, Potel J: Pharmakologische Untersuchungen mit Alkylsulfonyloxyalkyl- bzw. Chloräthyl-substituierten Oxazaphosphorin-2-oxiden. Arzneim-Forsch Drug Res 24:1149–1160, 1974
Voelcker H, Haegelsperger R, Hohorst HJ: Fluorometrische Bestimmung von “aktiviertem” Cyclophosphamid und Ifosfamid im Blut. J Cancer Res Clin Oncol 93:223–240, 1979
Stekar J: Photometrische Bestimmung von Mesna und Dimesna in Blutplasma und Urin. Ärztl Lab 28:187–191, 1982
Brock N, Hohorst HJ: The problem of specificity and selectivity of alkylating cytostatics: studies on N-2-chloroethylamido-oxazaphosphorines. Z Krebsforsch 88:185–215, 1977
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pohl, J., Hilgard, P., Jahn, W. et al. Experimental toxicology of ASTA Z 7557 (INN mafosfamide). Invest New Drugs 2, 201–206 (1984). https://doi.org/10.1007/BF00232352
Issue Date:
DOI: https://doi.org/10.1007/BF00232352