Abstract
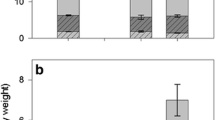
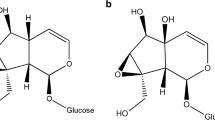
The iridoid glycoside content of individual adultEuphydryas anicia butterflies from two Colorado populations was quantitatively determined. At one site (Red Hill), larval host plants wereCastilleja integra andBesseya plantaginea, while at the other site (Cumberland Pass) a single host plant,B. alpina, was used. At Red Hill, macfadienoside and catalpol were sequestered, while at Cumberland Pass, catalpol and aucubin were sequestered. Artificial diet studies showed that larvae hydrolyzed a major iridoid ofB. plantaginea, 6-isovanilIylcatalpol, to catalpol (which was sequestered) and isovanillic acid (which was excreted). Large year-to-year and individual variation in butterfly iridoid content was established as was a female-male difference in macfadienoside vs. catalpol content. Larval host plant distributions and numbers were determined at Red Hill for two years and compared with changes in butterfly populations and sequestered iridoids.
Similar content being viewed by others
References
Andersen, J.M., andPedersen, W.B. 1983. Separation of phenolics and coumarins by reversedphase high-performance liquid chromatography.J. Chromatogr. 259:131–139.
Bowers, M.D. 1980. Unpalatability as a defense strategy ofEuphydryas phaeton (Lepidoptera: Nymphalidae).Evolution 34:586–600.
Bowers, M.D. 1981. Unpalatability as a defense strategy of western checkerspot butterflies (Euphydryas, Nymphalidae).Evolution 35:367–375.
Bowers, M.D. 1983. Iridoid glycosides and larval host-plant specificity in checkerspot butterflies (Euphydryas, Nymphalidae).J. Chem. Ecol. 9:475–493.
Bowers, M.D. 1986. Population differences in larval hostplant use in a checkerspot butterfly.Entomol. Exp. Appl. 40:61–69.
Bowers, M.D., andPuttick, G.M. 1986. The fate of ingested plant allelochemicals: Iridoid glycosides and lepidopteran herbivores.J. Chem. Ecol. 12:169–178.
Brower, L.P. 1984. Chemical defense in butterflies, pp. 109–139,in R.I. Vane-Wright and P.R. Ackery, (eds.). The Biology of Butterflies. Academic Press, New York.
Brower, L.P., Seiber, J.N., Nelson, C.J., Lynch, S.P., Hoggard, M.P., andCohen, J.A., 1984. Plant-determined variation in cardenolide content and thin-layer chromatography profiles of monarch butterflies,Danaus plexippus, reared on milkweed plants in California. 3.Asclepias californicn.J. Chem. Ecol. 10:1823–1857.
Brown, K.S., Jr. 1984a. Adult-obtained pyrrolizidine alkaloids defend ithomiine butterflies against a spider predator.Nature 309:707–709.
Brown, K.S., Jr. 1984b. Chemical ecology of dehydropyrrolizidine alkaloids in adult ithomiinae (Lepidoptera: Nymphalidae).Rev. Brasil. Biol. 44:435–460.
Cameron, D.W., Feutrill, G.I., Perlmutter, P., andSasse, J.M. 1984. Iridoids ofGarrya elliptica as plant growth inhibitors.Phytochemistry 23:533–535.
Casteele, K.V., Geiger, H., andSumere, C.F. 1983. Analysis of plant phenolics by high-performance liquid chromatography.J. Chromatogr. 258:111–124.
Cohen, J.A. 1985. Differences and similarities in cardenolide contents of queen and monarch butterflies in Florida and their ecological and evolutionary implications.J. Chem. Ecol. 11:85–103.
Cullenward, M.J., Ehrlich, P.R., White, R.R., andHoldren, C.E. 1979. The ecology and population genetics of an alpine checkerspot butterfly,Euphydryas anicia.Oecologia 38:1–12.
Davini, E., Iavarone, C., Trogolo, C., Aureli, P., andPasolini, B. 1986. The quantitative isolation and antimicrobial activity of the aglycone of aucubin.Phytochemistry 25:2420–2422.
Fox, L.R., andMorrow, P.A. 1981. Host specialization: Species property or local phenomenon?Science 211:888–893.
Franke, A., Rimpler, H., andSchneider, D. 1987. Iridoid glycosides in the butterflyEuphydryas cynthia (Lepidoptera, Nymphalidae).Phytochemistry 26:103–106.
Gardner, D. 1987. Iridoid chemistry of someCastilleja andBesseya plants and their hosted checkerspot butterflies. PhD thesis. Colorado State University.
Gardner, D.R., Narum, J., Zook, D., andStermitz, F.R. 1987. New iridoid glucosides fromCastilleja andBesseya: 6-hydroxyadoxoside and 6-isovanillylcatalpol.J. Nat. Prod. 50:485–489.
Holdren, C.E., andEhrlich, P.R. 1982. Ecological determinants of food plant choice in the checkerspot butterflyEuphydryas editha in Colorado.Oecologia 52:417–423.
Ishiguro, K., Yamaki, M., Takagi, S., Ikeda, Y., Kawakami, K., Ito, K., andNose, T. 1986. Studies on iridoid-related compounds. IV. Antitumor activity of iridoid aglycones.Chem. Pharm. Bull. 34:2375–2379.
Jones, C.G., Hess, T.A., Whitman, D.W., Silk, P.J., andBlum, M.S. 1986. Idiosyncratic variation in chemical defenses among individual generalist grasshoppers.J. Chem. Ecol. 12:749–761.
Lynch, S.P., andMartin, R.A. 1987. Cardenolide content and thin layer chromatographic profiles of monarch butterflies,Danaus plexippus L., and their larval host-plant milkweed,Asclepias viridis Walt, in northwestern Louisiana.J. Chem. Ecol. 13:47–70.
Murphy, D.D., Launer, A.E., andEhrlich, P.R. 1983. The role of adult feeding in egg production and population dynamics of the checkerspot butterflyEuphydryas editha.Oecologia 56:257–263.
Odendaal, F.J.,Turchin, P., andStermitz, F.R. 1988a. An incidental-effect hypothesis explaining aggregation of males in a population ofEuphydryas anicia (Lepidoptera).Am. Nat. In press.
Odendaal, F.J.,Turchin, P., andStermitz, F.R. 1988b. Host distribution and male harassment influence the distribution of femaleEuphydryas anicia in Colorado.Oecologia. In press.
Pollard, E. 1977. A method for assessing change in the abundance of butterflies.Biol. Conserv. 12:115–134.
Scudder, G.G.E., Moore, L.V., andIsman, M.B. 1986. Sequestration of cardenolides inOncopeltus fasciatus: Morphological and physiological adaptations.J. Chem. Ecol. 12:1171–1187.
Singer, M.C. 1983. Determinants of multiple host use in a phytophagous insect population.Evolution 37:389–403.
Stanton, M.L., andCook, R.E. 1984. Sources of intraspecific variation in the hostplant seeking behavior ofColias butterflies.Oecologia 61:265–270.
Stermitz, F.R., Gardner, D.R., Odendaal, F.J., andEhrlich, P.R. 1986.Euphydryas anicia (Lepidoptera: Nymphalidae) utilization of iridoid glycosides fromCastilleja andBesseya (Scrophulariaceae) hostplants.J. Chem. Ecol. 12:1459–1468.
White, R.R. 1979. Foodplant of alpineEuphydryas anicia (Nymphalidae).J. Lepid. Soc. 33:170–173.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gardner, D.R., Stermitz, F.R. Host plant utilization and iridoid glycoside sequestration byEuphydryas anicia (Lepidoptera: Nymphalidae). J Chem Ecol 14, 2147–2168 (1988). https://doi.org/10.1007/BF01014022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01014022