Abstract
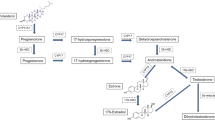
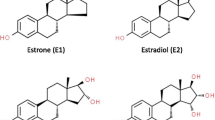
Estrogen action in the target cells is dependent on estrogen receptor activity and intracellular estrogen concentration, which, in turn, is affected by the serum concentration and local metabolism in these cells. During the reproductive years the main source of estrogens is the ovarian follicles, but in postmenopausal women most of the estrogens are formed in peripheral tissues. 17β-hydroxysteroid dehydrogenases (17HSDs)6 catalyze the reaction between 17β-hydroxysteroids and 17-ketosteroids, and several distinct 17HSD isoenzymes have been characterized. 17HSD type 1 catalyzes the reaction from low-activity estrone to high-activity estradiol. The type 2 enzyme has an opposite activity, thereby reducing the exposure of tissues to estrogen action. 17HSD type 1 is expressed both in steroidogenic tissues and in the target tissues of steroid action, such as normal and malignant breast tissue, where it may be responsible for maintaining the high intracellular estradiol concentration seen in breast cancer specimens. Therefore, 17HSD type 1 inhibitors may be useful in the treatment and/or prevention of estrogen-dependent malignancies, such as breast cancer. This article deals mainly with 17HSD types 1 and 2 and their role in estrogen action in breast tissue.
Similar content being viewed by others
REFERENCES
J. S. Richards (1980). Maturation of ovarian follicles: Actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol. Rev. 60:51–89.
J. Russo, B. A. Gusterson, A. E. Rogers, I. H. Russo, S. R. Wellings, and J. van Zwieten (1990). Comparative study of human and rat mammary tumorigenesis. Lab. Invest. 62:244–278.
G. J. Pepe and E. D. Albrecht (1995). Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Rev. 16:608–648.
E. Perel, M. E. Blackstein, and D. W. Killinger (1982). Aromatase in human breast carcinoma. Cancer Res. 42:3369s–3372s.
H. Sasano and N. Harada (1998). Intratumoral aromatase in human breast, endometrial, and ovarian malignancies. Endocrine Rev. 19:593–607.
J. R. Pasqualini, C. Celly, B.-L. Nguyen, and C. Vella (1989). Importance of estrogen sulfates in breast cancer. J. Steroid Biochem. 34:155–163.
S. J. Santner, P. D. Feil, and R. J. Santen (1984). In situ estrogen production via the estrone sulfatase pathway in breast tumors: Relative importance versus the aromatase pathway. J. Clin. Endocrinol. Metab. 59:29–33.
B. Longcope (1996). Dehydroepiandrosterone metabolism. J. Endocrinol. 150:S125–S127.
F. Labrie, V. Luu-The, S.-X. Lin, C. Labrie, J. Simard, R. Breton, and A. Bélanger (1997). The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62:148–158.
A. Brodie, Q. Lu, and J. Nakamura (1997). Aromatase in the normal breast and breast cancer. J. Steroid Biochem. Mol. Biol. 61:281–286.
S. J. Santner, D. Leszczynski, C. Wright, A. Manni, P. D. Feil, and R. J. Santen (1986). Estrone sulfate: A potential source of estradiol in human breast cancer tissues. Breast Cancer Res. Treat. 7:35–44.
M. Poutanen, V. Isomaa, H. Peltoketo, and R. Vihko (1995). Role of 17β-hydroxysteroid dehydrogenase type 1 in endocrine and intracrine estradiol biosynthesis. J. Steroid Biochem. Mol. Biol. 55:525–532.
A. A. J. Van Landeghem, J. Poortman, M. Nabuurs, and J. H. H. Thijssen (1985). Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res. 45: 2900–2906.
A. Vermeulen, J. P. Deslypere, and R. Paridaens (1986). Steroid dynamics in the normal and carcinomatous mammary gland. J. Steroid Biochem. 25:799–802.
R. C. Bonney, M. J. Reed, P. A. Beranek, M. W. Ghilchik, and V. H. T. James (1986). Metabolism of [3H]oestradiol in vivo by normal breast and tumor tissue in postmenopausal women. J. Steroid Biochem. 24:361–364.
J. H. H. Thijssen, M. A. Blankenstein, W. R. Miller, and A. Milewicz (1987). Estrogens in tissues: Uptake from the peripheral circulation or local production. Steroids 50:297–306.
F. P. Li, J. A. Schneider, and A. F. Kantor (1993). Cancer epidemiology. Cancer Med. 1:332–339.
R. Vihko and D. Apter (1989). Endogenous steroids in the pathophysiology of breast cancer.CRC Crit. Rev Oncol/Hematol 9:1–16.
C. M. Mansfield (1993). A review of the etiology of breast cancer. J. Natl. Med. Assoc. 85:217–221.
L. Lipworth (1995). Epidemiology of breast cancer. Eur. J. Cancer. Prev. 4:7–30.
J. A. Cauley, J. P. Gutai, L. H. Kuller, D. LeDonne, and J. G. Powell (1989). The epidemiology of serum sex hormones in postmenopausal women. Am. J. Epidemiol. 129:1120–1131.
J. L. Kelsey and L. Bernstein (1996). Epidemiology and prevention of breast cancer. Ann. Rev. Publ. Health 17:47–67.
H. Spencer Feigelson and B. E. Henderson (1996). Estrogens and breast cancer. Carcinogenesis 17:2279–2284.
R. J. B. King (1991). A discussion of the roles of oestrogen and progestin in human mammary carcinogenesis. J. Steroid. Biochem. Mol. Biol. 39:811–818.
M. E. Lippman and R. B. Dickson (1989). Mechanisms of normal and malignant breast epithelial growth regulation. J. Steroid Biochem. 34:107–121.
R. J. Santen and H. Harvey (1999). Use of aromatase inhibitors in breast carcinoma Endocrine-Related Cancer 6:75–92.
H. Peltoketo, P. Vihko, and R. Vihko (1999). Regulation of estrogen action: Role of 17β-hydroxysteroid dehydrogenases. Vitamin Horm. 55:353–397.
N. Moghrabi, J. R. Head, and S. Andersson (1997). Cell typespecific expression of 17β-hydroxysteroid dehydrogenase type 2 in human placenta and fetal liver. J. Clin. Endocrinol. Metab. 82:3872–3878.
M. V. J. Mustonen, M. H. Poutanen, S. Kellokumpu, Y. de Launoit, V. V. Isomaa, R. K. Vihko, and P. T. Vihko (1998). Mouse 17β-hydroxysteroid dehydrogenase type 2 mRNA is predominantly expressed in hepatocytes and in surface epithelial cells of the gastrointestinal and urinary tracts. J. Mol. Endocrinol. 20:67–74.
H. Peltoketo, V. Luu-The, J. Simard, and J. Adamski (1999). 17β-Hydroxysteroid dehydrogenase (HSD)/17-ketosteroid reductase (KSR) family; Nomenclature and main characteristics of the 17HSD/KSR enzymes. J. Mol. Endocrinol. 23:1–11.
A. Krazeisen, R. Breitling, K. Imai, S. Fritz, G. Möller, and J. Adamski (1999). Determination of cDNA, gene structure and chromosomal localization of the novel human 17β-hydroxysteroid dehydrogenase type 7. FEBS Lett. 460:373–379.
Y.-M. Qin, M. H. Poutanen, H. M. Helander, A.-P. Kvist, K. M. Siivari, W. Schmitz, E. Conzelmann, U. Hellman, and K. Hiltunen (1997). Peroxisomal multifunctional enzyme of β-oxidation metabolizing D-3-hydroxyacyl-CoA esters in rat liver: Molecular cloning, expression and characterization. Biochem. J. 321:21–28.
S. A. Ghersevich, M. H. Poutanen, H. K. Martikainen, and R. K. Vihko (1994). Expression of 17β-hydroxysteroid dehydrogenase type 1 in human granulosa cells: Correlation with follicular size, cytochrome P450 aromatase activity and oestradiol production. J. Endocrinol. 143:139–150.
O. Mäentausta, R. Sormunen, V. Isomaa, V.-P. Lehto, P. Jouppila, and R. Vihko (1991). Immunohistochemical localization of 17β_-hydroxysteroid dehydrogenase in the human endometrium during the menstrual cycle. Lab. Invest. 65:582–587.
W. M. Geissler, D. L. Davis, L. Wu, K. D. Bradshaw, S. Patel, B. B. Mendoca, K. O. Elliston, J. D. Wilson, D. W. Russell, and S. Andersson (1994). Male pseudohermaphroditism caused by mutations of testicular 17β-hydroxysteroid dehydrogenase 3. Nat. Genet. 7:34–39.
J. A. Sha, K. Dudley, W. R. A. K. J. S. Rajapaksha, and P. J. O'shaughnessy (1997). Sequence of mouse 17β-hydroxysteroid dehydrogenase type 3 cDNA and tissue distribution of the type 1 and type 3 isoform mRNAs. J. Steroid Biochem. Mol. Biol. 60:19–24.
Y. Deyashiki, K. Ohshima, M. Nakanishi, K. Sato, K. Matsuura, and A. Hara (1995). Molecular cloning and characterization of mouse estradiol 17β-dehydrogenase (A-specific), a member of the aldoketoreductase family. J. Biol. Chem. 270:10461–10467.
M. G. Biswas and D. W. Russel (1997). Expression cloning and characterization of oxidative 17β-and 3β-hydroxysteroid dehydrogenases from rat and human prostate. J. Biol. Chem. 272:15959–15966.
P. Nokelainen, H. Peltoketo, R. Vihko, and P. Vihko (1998). Expression cloning of a novel estrogenic mouse 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase (m17HSD7), previously described as a prolactin receptor-associated protein (PRAP) in rat. Mol. Endocrinol. 12:1048–1059.
J. Fomitcheva, M. E. Baker, E. Anderson, G. Y. Lee, and N. Aziz (1998). Characterization of Ke 6, a new 17β-hydroxysteroid dehydrogenase, and its expression in gonadal tissues. J. Biol. Chem. 273:22664–22671.
M. L. Casey, P. C. MacDonald, and S. Andersson (1994). 17β-Hydroxysteroid dehydrogenase type 2: Chromosomal assignment and progestin regulation of gene expression in human endometrium. J. Clin. Invest. 94:2135–2141.
M. V. J. Mustonen, M. H. Poutanen, V. V. Isomaa, R. K. Vihko, and P. T. Vihko (1997). Cloning of mouse 17β-hydroxysteroid dehydrogenase type 2, and analyzing expression of themRNAs for types 1, 2, 3, 4, and 5 in mouse embryos and adult tissues. Biochem. J. 325:199–205.
M. V. J. Mustonen, V. V. Isomaa, T. Vaskivuo, J. Tapanainen, M. H. Poutanen, F. Stenbäck, R. K. Vihko, and P. T. Vihko (1998). Human 17β-hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression and localization in term placenta and in endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 83:1319–1324.
J. Adamski, T. Normand, F. Leenders, D. Monté, A. Begue, D. Stéhelin, P. W. Jungblut, and Y. de Launoit (1995). Molecular cloning of a novel widely expressed human 80 kDa 17β-hydroxysteroid dehydrogenase IV. Biochem. J. 311:437–443.
M. Poutanen, V. Isomaa, V.-P. Lehto, and R. Vihko (1992). Immunological analysis of 17β-hydroxysteroid dehydrogenase in benign and malignant human breast tissue. Int. J Cancer 50:386–390.
M. Poutanen, B. Moncharmont, and R. Vihko (1992). 17β-Hydroxysteroid dehydrogenase gene expression in human breast cancer cells: Regulation of expression by a progestin. Cancer Res. 52:290–294.
M. M. Miettinen, M. V. J. Mustonen, M. H. Poutanen, V. V. Isomaa, and R. K. Vihko (1996). Human 17β-hydroxysteroid dehydrogenase type 1 and type 2 isoenzymes have opposite activities in cultured cells and characteristic cell-and tissuespecific expression. Biochem. J. 314:839–845.
M. Poutanen, V. Isomaa, K. Kainulainen, and R. Vihko (1990). Progestin induction of 17β-hydroxysteroid dehydrogenase enzyme protein in the T-47D human breast-cancer cell line. Int. J. Cancer 46:897–901.
E. F. Adams, B. Rafferty, and M. C. White (1991). Interleukin 6 is secreted by breast fibroblasts and stimulates 17β-oestradiol oxidoreductase activity of MCF-7 cells: Possible paracrine regulation of breast 17β-oestradiol levels. Int. J. Cancer. 49:118–121.
L. J. Duncan, N. G. Goldham, and M. J. Reed (1994). The interaction of cytokines in regulating oestradiol 17β-hydroxysteroid dehydrogenase activity in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 49:63–68.
A. Singh and M. J. Reed (1991). Insulin-like growth factor type I and insulin-like growth factor type II stimulate oestradiol-17β hydroxysteroid dehydrogenase (reductive) activity in breast cancer cells. J. Endocrinol. 129:R5–R8.
M. J. Reed, D. Rea, L. J. Duncan, and M. G. Parker (1994). Regulation of estradiol 17β-hydroxysteroid dehydrogenase expression and activity by retinoic acid in T47D breast cancer cells. Endocrinology 135:4–9.
Y.-S. Piao, H. Peltoketo, A. Jouppila, and R. Vihko (1997). Retinoic acids increase 17β-hydroxysteroid dehydrogenase type 1 expression in JEG-3 and T47D cells, but the stimulation is potentiated by epidermal growth factor, 12-O-tetradecanoylphorbol-13-acetate, and cyclic adenosine 3′,5′-monophosphate only in JEG-3 cells. Endocrinology 138:898–904.
M. M. Miettinen, M. H. Poutanen, and R. K. Vihko (1996). Characterization of estrogen-dependent growth of cultured MCF-7 human breast-cancer cells expressing 17β-hydroxysteroid dehydrogenase type 1. Int. J Cancer 68:600–604.
V. Luu The, C. Labrie, H. F. Zhao, J. Couët, Y. Lachance, J. Simard, G. Leblanc, J. Côté, D. Bérubé, R. Gagné, and F. Labrie (1989). Characterization of cDNAs for human estradiol 17β-dehydrogenase and assignment of the gene to chromosome 17: Evidence of two mRNA species with distinct 5′-termini in human placenta. Mol. Endocrinol. 3:1301–1309.
V. Luu-The, C. Labrie, J. Simard, Y. Lachance, H.-F. Zhao, J. Couët, G. Leblanc, and F. Labrie (1990). Structure of two in tandem human 17β-hydroxysteroid dehydrogenase genes. Mol. Endocrinol. 4:268–275.
H. Peltoketo, V. Isomaa, and R. Vihko (1992). Genomic organization and DNA sequences of human 17β-hydroxysteroid dehydrogenase dgenes and flanking regions. Eur. J. Biochem. 209:459–466.
Y. Tremblay, G. E. Ringler, Y. Morel, T. K. Mohandas, F. Labrie, J. F. Strauss, III, and W. L. Miller (1989). Regulation of the gene for estrogenic 17-ketosteroid reductase lying on chromosome 17cen→q25. J. Biol. Chem. 264:20458–20462.
Y.-S. Piao, H. Peltoketo, P. Vihko, and R. Vihko (1997). The proximal promoter region of the gene encoding human increase 17β-hydroxysteroid dehydrogenase type 1 contains GATA, AP-2 and Sp1 response elements: Analysis of promoter function in chorioncarcinoma cells. Endocrinology 138:3417–3425.
Y.-S. Piao, H. Peltoketo, J. Oikarinen, and R. Vihko (1995). Coordination of transcription of the human 17β-hydroxysteroid dehydrogenase type 1 gene (EDH17B2) by a cell-specific enhancer and a silencer: Identification of retinoic acid response element. Mol. Endocrinol. 9:1633–1644.
S. Leivonen, Y.-S. Piao, H. Peltoketo, P. Numchaisrika, R. Vihko, and P. Vihko (1999). Identification of essential subelements in the hHSD17B1 enhancer: Difference in function of the enhancer and that of the hHSD17BP1 analog is due to –480C and –486G. Endocrinology 140:3478–3487.
D. Ghosh, V. Z. Pletnev, D.-W. Zhu, Z. Wawrzak, W. L. Duax, W. Pangborn, F. Labrie, and S.-X. Lin (1995). Structure of human estrogenic 17β-hydroxysteroid dehydrogenase at 2. 20Å resolution. Structure 3:503–513.
M. Poutanen, M. Miettinen, and R. Vihko (1993). Differential estrogen specificities for transiently expressed human placental 17β-hydroxysteroid dehydrogenase and an endogenous enzyme expressed in cultured COS-m6 cells. Endocrinology 133:2639–2644.
T. Puranen, M. Poutanen, D. Ghosh, P. Vihko, and R. Vihko (1997). Characterization of structural and functional properties of human 17β-hydroxysteroid dehydrogenase type 1 using recombinant enzymes and site-directed mutants. Mol. Endocrinol. 11:77–86.
T. Puranen, M. Poutanen, D. Ghosh, R. Vihko, and P. Vihko (1997). Origin of substrate specificity of human and rat 17β-hydroxysteroid dehydrogenase type 1, using chimeric enzymes and site-directed substitutions. Endocrinology 138:3532–3539.
A. Azzi, P. H. Rehse, D. W. Zhu, R. L. Campabell, F. Labrie, and S.-X. Lin (1996). Crystal structure of human estrogenic 17β-hydroxysteroid dehydrogenase complexed with 17β-estradiol. Nat. Struct. Biol. 3(8):665–668.
R. Breton, D. Housset, C. Mazza, and J. C. Fontecill-Camps (1996). The structure of a complex of human 17β-hydroxysteroid dehydrogenase with estradiol and NADP+ identifies two principal targets for the design of inhibitors. Structure 4:905–915.
S.-X. Lin, D.-W. Zhu, A. Azzi, R. L. Campbell, R. Breton, F. Labrie, D. Ghosh, V. Pletnev, W. L. Duax, and W. Pangborn (1996). Studies on the three-dimensional structure of estrogenic 17β-hydroxysteroid dehydrogenase. J. Endocrinol. 150:S13–S20.
C. Mazza, R. Breton, D. Housset, and J. C. Fontecilla-Camps (1998). Unusual charge stabilization of NADP+ in 17β-hydroxysteroid dehydrogenase. J. Biol. Chem. 273:8145–8152.
H. Jörnvall, B. Persson, M. Krook, S. Atrian, R. Conzáles-Duarte, J. Jeffery, and D. Ghosh (1995). Short-chain dehydrogenases/reductases (SDR). Biochemistry 34:6003–6013.
D. Ghosh, C. M. Weeks, P. Grochulski W. L. Duax, M. Erman, R. L. Rimsay, and J. C. Orr (1991). Three-dimensional structure of holo 3α,20β-hydroxysteroid dehydrogenase:Amember of the short-chain dehydrogenase family. Proc. Natl. Acad. Sci. U.S.A. 88:10064–10068.
D. Ghosh, Z. Wawrzak, C. M. Weeks, W. L. Duax, and M. Erman (1994). The refined three-dimensional structure of 3α,20β-hydroxysteroid dehydrogenase and possible roles of the residues conserved in short-chain dehydrogenases. Structure 2:629–640.
T. M. Penning (1996). 17β-Hydroxysteroid dehydrogenase: Inhibitors and inhibitor design. Endocrine-Related Cancer 3:41–56.
M. W. Sawicki, M. Erman, T. Puranen, P. Vihko, and D. Ghosh (1999). Structure of the ternary complex of human 17β-hydroxysteroid dehydrogenase type 1 with 3-hydroxyestra-1,3,5,7-tetraen-17-one (equilin) and NADP+. Proc. Natl. Acad. Sci. U.S.A. 96:840–845.
M. W. Sawicki, N. Li, and D. Ghosh (1999). Equilin. Acta Crystallogr. 15:425–427.
L. Wu, M. Einstein, W. M. Geissler, H. K. Chan, K. O. Elliston, and S. Andersson (1993). Expression cloning and characterization of human 17β-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20α-hydroxysteroid dehydrogenase activity. J. Biol. Chem. 268:12964–12969.
L. A. Akinola, M. Poutanen, and R. Vihko (1996). Cloning of rat 17β-hydroxysteroid dehydrogenase type 2 and characterization of tissue distribution and catalytic activity of rat type 1 and type 2 enzymes. Endocrinology 137:1572–1579.
J. P. Elo, L. A. Akinola, M. Poutanen, P. Vihko, A. P. Kyllö-nen, O. Lukkarinen, and R. Vihko (1996). Characterization of 17β-hydroxysteroid dehydrogenase isoenzyme expression in benign and malignant human prostate. Int. J. Cancer 66:37–41.
G. H. Tait, C. J. Newton, M. J. Reed, and V. H. T. James (1989). Multiple forms of 17β-hydroxysteroid oxidoreductase in human breast tissue. J. Mol. Endocrinol. 2:71–80.
V. Z. Mann, C. J. Newton, and G. H. Tait (1991). 17β-hydroxysteroid dehydrogenases in human breast tissues: Puri-fication and characterization of soluble enzymes and the distibution of particulate and soluble enzymes in adipose, nonadipose and tumor tissues. J. Mol. Endocrinol. 7:45–55.
M. Miettinen, M. Mustonen, M. Poutanen, V. Isomaa, M. Wickman, G. Söderqvist, R. Vihko, and P. Vihko (1999). 17β-Hydroxysteroid dehydrogenases in normal human mammary epithelial cells and breast tissue. Breast Cancer Res. Treat. 57:175–182.
W. R. Duan, D. I. H. Linzer, and G. Gibori (1996). Cloning and characterization of an ovarian specific protein that associates with the short form of the prolactin receptor. J. Biol. Chem. 271:15602–15607.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miettinen, M., Isomaa, V., Peltoketo, H. et al. Estrogen Metabolism as a Regulator of Estrogen Action in the Mammary Gland. J Mammary Gland Biol Neoplasia 5, 259–270 (2000). https://doi.org/10.1023/A:1009542710520
Issue Date:
DOI: https://doi.org/10.1023/A:1009542710520