Abstract
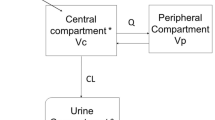
The pharmacokinetics of teicoplanin, a new glycopeptide antibiotic active against gram-positive aerobic and anaerobic bacteria, was studied in adult male volunteers given 2- and 3- mg/ kg doses by a constant-rate 0.5-hr infusion. Serum and urine samples were collected up to 96 hr. Mean peak serum levels after the two doses were 15.7 and 22.4 μg/ml. Postinfusion serum teicoplanin levels showed triexponential decay. A three-compartment body model gave close values for pharmacokinetic parameters after the two doses. The mean half-life of the λ1 phase was 20.3 min, that of the λ2 phase was 2.9 hr, and the half-life of the estimated λ3 phase was 40.5 hr, in good agreement with that of the λZ phase (45.9 hr) calculated from the last urine data. The mean volume of distribution of the central compartment was 0.09 liter/kg and the steady-state volume of distribution using noncompartmental analysis was 0.84 liter/kg. Total clearance averaged 16.05 ml/hr/kg, with renal clearance arbout half this (9.51 ml/hr/kg), calculated by two different methods. The average total recovery of active teicoplanin in urine over 4 days was 52%, suggesting that both renal and nonrenal mechanisms are involved in elimination of the drug. The concentrations of teicoplanin in serum and urine exceeded the MIC (ranging from 0.02 to 2 μg/ml) on many pathogenic organisms for at least 1 day after administration.
Similar content being viewed by others
References
F. Parenti, G. Beretta, M. Berti, and V. Arioli. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. I. Description of the producer strain, fermentation studies and biolgical properties.J. Antibiot. 31:276–283 (1978).
M. H. Cynamon and P. A. Granato. Comparison of thein vitro activities of teichomycin A2 and vancomycin against staphylococci and enterococci.Antimicrob. Agents Chemother. 21:504–505 (1982).
M. Berti, R. Pallanza, and V. Arioli. Teichomycin A2: A new antibiotic from Actinoplanes. Activityin vitro andin vivo. InCurrent Chemotherapy and Immunotherapy, P. Periti and G. Gialdroni Grassi (eds.). American Society for Microbiology, Washington, D.C., 1982, pp. 342–343.
S. Somma and L. Gastaldo. Mechanism of action of teichomycin A2. A new antibiotic. InCurrent Chemotherapy and Immunotherapy, P. Periti and G. Gialdroni Grassi (eds.). American Society for Microbiology, Washington, D. C., 1982, pp. 343–345.
L. Verbist and B. Hendrickx. Comparative activity of teichomycin and 11 other antibiotics against gram-positive cocci. In Abstracts, 5th International Symposium on Future Trends in Chemotherapy, Tirrenia, Pisa, May 1982, p. 88.
P. E. Varaldo and G. C. Schito. Teichomycin:In vitro antibacterial activity against streptococci and staphylococci and comparison with vancomycin. In Abstracts, 5th International Symposium on Future Trends in Chemotherapy, Tirrenia, Pisa, May 1982, p. 87.
R. N. Gruneberg, G. L. Ridgway, A. L. Cremer, and D. Felmingham. The sensitivity of gram positive pathogens to teichomycin and to vancomycin. In Abstracts, 5th International Symposium on Future Trends in Chemotherapy, Tirrenia, Pisa, May 1982, p. 86.
M. Gibaldi and D. Perrier.Pharmacokinetics. Marcel Dekker, New York, 1975.
K. Yamaoka, T. Nakagawa, and T. Uno. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations.J. Phannacokinet. Biopharm. 6:165–175 (1978).
H. G. Boxenbaum, S. Riegelman, and R. M. Elashoff. Statistical estimations in pharmacokinetics.J. Pharmacokinet. Biopharm. 2:123–148 (1974).
L. Z. Benet and R. L. Galeazzi. Noncompartmental determination of the steady-state volume of distribution.J. Pharm. Sci. 68:1071–1074 (1979).
S. Riegelman and P. Collier. The application of statistical moment theory to the evaluation ofin vivo dissolution time and absorption time.J. Pharmacokinet. Biopharm. 8:509–534 (1980).
K. K. H. Chan and M. Gibaldi. Estimation of statistical moments and steady-state volume of distribution for a drug given by intravenous infusion.J. Pharmacokinet. Biopharm. 10:551–558 (1982).
R. Platzer, G. Reutemann, and R. L. Galeazzi. Pharmacokinetics of intravenous isorbidedinitrate.J. Pharmacokinet. Biopharm. 10:575–586 (1982).
M. Rocchetti and M. Recchia. SPBS: Statistical programs for biological sciencesMinicomputer software for applying routine biostatistical methods.Comp. Prog. Biomed. 14:7–20 (1982).
Author information
Authors and Affiliations
Additional information
This work was supported in part by a grant from Gruppo Lepetit, Milan, Italy, and by a CNR (National Research Council, Rome, Italy) grant on “Clinical Pharmacology and Rare Diseases.”
Rights and permissions
About this article
Cite this article
Traina, G.L., Bonati, M. Pharmacokinetics of teicoplanin in man after intravenous administration. Journal of Pharmacokinetics and Biopharmaceutics 12, 119–128 (1984). https://doi.org/10.1007/BF01059273
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059273