Abstract
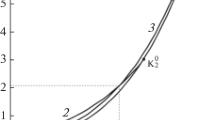
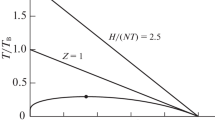
A simple statistical mechanical theory is presented to explain phase diagrams of fluid mixtures with both a lower critical solution temperature and an upper critical solution temperature under pressure. By postulating a temperature dependence for the interaction free energy parameter of the constituent molecules and a pressure dependence for the excess volume, phase diagrams with both lower critical solution temperature, and upper critical solution temperature and their pressure dependence can be reproduced by quadratic surfaces in temperature-concentration-pressure space. The topological aspects of the observed phase diagrams in this space have been related to our theoretical model, and the thermodynamical meaning of the topologies has been interpreted based on our model. Experimental data for the mutual solubility of water and 2-butanol under pressure and that of water and 3-methylpyridine with added salts have been analyzed quantitatively and theoretical parameters are determined.
Similar content being viewed by others
References
W. J. Moore,Physical Chemistry, 4th ed., Chap. 7 (Prentice-Hall, Englewood Cliffs, NJ, 1972).
E. A. Guggenheim,Thermodyanics, Chap. 4, (North Holland, Amsterdam, 1967).
R. Fowler and E. A. Guggenheim,Statistical Thermodynamics, Chap. 8 (Cambridge University Press, Cambridge, 1952).
W. L. Bragg and E. J. Williams,Proc. Roy. Soc. A145, 699 (1934).
H. A. Bethe,Proc. Roy. Soc. A150, 552 (1935).
J. A. Barker and W. Fock,Disc. Faraday Soc. 15, 188 (1953).
J. C. Wheeler,J. Chem. Phys. 62, 433 (1975).
G. R. Andersen and J. C. Wheeler,J. Chem. Phys. 69, 2082 (1978).
G. M. Schneider, inWater, A Comprehensive Treatise, P. Franks, ed. (Plenum Press, New York, 1973), p. 381.
G. M. Schneider,Chem. Thermodyn. 2, 105 (1978).
G. M. Schneider,Fortschr. Chem. Forsch. 13, 559 (1970).
T. Moriyoshi, S. Kaneshina, and K. Yabumoto,J. Chem. Thermodyn. 7, 537 (1975).
K. Nakanishi, K. Ashitani, and H. Touhara,J. Chem. Thermodyn. 8, 121 (1976).
J. Abe, K. Nakanishi, and H. Touhara,J. Chem. Thermodyn. 10, 483 (1978).
D. Eisenberg and W. Kauzmann,The Structure and Properties of Water, Chap. 3 (Clarendon Press, Oxford, 1969).
K. Nakanishi,Bull. Chem. Soc. Jap. 33, 793 (1960).
G. M. Schneider,Ber. Bunsen. Phys. Chem. 70, 497 (1966).
C. V. Krishnan and H. L. Friedman,J. Solution Chem. 3, 727 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Suezaki, Y., Kaneshina, S., Shirahama, K. et al. A simple statistical mechanical theory for the mutual solubility of fluid mixtures of water and organic solvents under high pressure. J Solution Chem 17, 637–651 (1988). https://doi.org/10.1007/BF00645975
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00645975