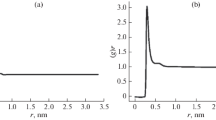
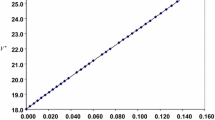
Abstract
The densities and the ultrasonic speeds of the aqueous solutions of 2-(2-hexyloxyethoxy)ethanol (C6E2) were measured over the entire range of mole fractions at 5°C. Excess molar volumes VE were readily calculated from the densities. The densities, in combination with the ultrasonic speeds, furnish estimates of the molar (and excess molar) isentropic compressibilities K S and the deviations uD of the ultrasonic speeds from the values calculated for ideal mixtures. Radical changes in the mole fraction derivatives of the excess molar properties of the (C6E2 + water) system, in the vicinity of an amphiphile mole fraction of 0.003, indicate that C6E2 like C6E3 is capable of micelle formation. Our data have been compared with those reported earlier for (C4E2 +, C2E2 +, and C6E3 + water). We have employed both mass action and pseudophase approaches to data analysis, together with the four-segment model approach.
Similar content being viewed by others
References
G. Douhéret, A. Pal, and M. I. Davis,J. Chem. Thermodyn. 22, 99 (1990).
G. Douhéret, C. Salgado, M. I. Davis, and J. Loya,Thermochimica Acta 207, 313 (1992).
G. Roux, G. Perron, and J. E. Desnoyers,J. Solution Chem 7, 639 (1978).
V. Degiorgio, inPhysics of Amphiphiles, Micelles, Vesicles and Microemulsions, V. Degiorgio and M. Conti, eds., (North Holland, Amsterdam, 1985), p. 303.
M. Costas, personal communication.
G. Douhéret and M. I. Davis,Chem. Soc. Revs 22, 43 (1993).
IUPAC,Pure Appl. Chem. 58, 1677 (1986).
P. Picker, E. Tremblay, and C. Jolicoeur,J. Solution Chem. 3, 377 (1974).
G. S. Kell,J. Chem. Eng. Data 20, 97 (1975).
M. Greenspan and C. E. Tschiegg,Rev. Sci. Instr. 28, 897 (1957).
V. A. Del Grosso and C. W. Mader,J. Acoust. Soc. 52, 1442 (1972).
G. Douhéret, A. Khadir, and A. Pal,Thermochimica Acta 142, 219 (1989).
J. S. Rowlinson,Liquids and Liquid Mixtures (Butterworths, London, 1959), p. 17.
M. I. Davis,Chem. Soc. Revs 22, 127 (1993).
G. Douhéret, C. Moreau, and A. Viallard,Fluid Phase Equil. 22, 289 (1985).
G. C. Benson and O. Kiyohara,J. Chem. Thermodyn. 11, 1061 (1979).
O. Kiyohara, C. J. Halpin, and G. C. Benson,Can. J. Chem. 57, 2335 (1979).
A. Aicart, M. Costas, E. Junquera, and G. Tardajos,J. Chem. Thermodyn 22, 1153 (1990).
W. J. de Haas,Procés-Verbaux Comité International Poids et Mesures 22, 85 (1950).
S. A. Wieczorek,J. Chem. Thermodyn. 24, 129 (1992).
O. Redlich and A. T. Kister,Ind. Eng. Chem. 40, 345 (1948).
A. H. Roux and J. E. Desnoyers,Proc. Indian Acad. Sci. (Chem. Sci.) 98, 435 (1987).
J. E. Desnoyers, G. Caron, R. DeLisi, D. Roberts, A. Roux, and G. Perron,J. Phys. Chem. 87, 1397 (1983).
G. Perron, L. Couture, and J. E. Desnoyers,J. Solution Chem. 21, 433 (1992).
K. Shinoda and E. Hutchinson,J. Phys. Chem. 66, 577 (1962).
M. I. Davis,Thermochimica Acta 77, 421 (1984).
M. I. Davis and G. Douhéret,Thermochimica Acta 188, 229 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Douhéret, G., Roux, A.H., Davis, M.I. et al. Thermodynamics of aqueous mixtures of 2-(2-Hexyloxyethoxy)ethanol at 5°C. J Solution Chem 22, 1041–1062 (1993). https://doi.org/10.1007/BF00647728
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00647728