Abstract
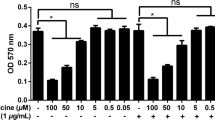
Previous studies revealed that expression and activation of cyclooxygenase-2 (Cox-2) conveyed a protective principle in murine macrophages, thus attenuating pro-apoptotic actions of chemotherapeutic agents or programmed cell death as a result of massive nitric oxide (NO) generation. Expression of Cox-2 was achieved by treatment of cells with lipopolysaccharide/interferon-γ or nontoxic doses of NO releasing agents. We reasoned E-type prostanoid formation, and in turn an intracellular cAMP increase as the underlying protective mechanism. To prove our hypothesis, we analyzed the effects of lipophilic cAMP-analogs on NO, cisplatin, or etoposide induced apoptosis in RAW 264.7 macrophages. Selected apoptotic parameters comprised DNA fragmentation (diphenylamine assay), annexin V staining of phosphatidylserine, caspase activity (quantitated by the cleavage of a fluorogenic caspase-3-like substrate Ac-DEVD-AMC), and mitochondrial membrane depolarisation (ΔΨ). Western blots detected accumulation of the tumor suppressor protein p53, relocation of cytochrome c to the cytosol, and expression of the anti-apoptotic protein Bcl-xL. Prestimulation with lipophilic cAMP-analogs attenuated apoptosis with the notion that cell death parameters were basically absent. To verify gene induction by cAMP in association with protection we established activation of cAMP response element binding protein (CREB) by gel-shift analysis and moreover, treated macrophages with oligonucleotides containing a cAMP-responsive element (CRE) in order to scavenge CREB. Decoy oligonucleotides, but not control oligonucleotides, attenuated cAMP-evoked protection and reestablished pro-apoptotic parameters. We conclude that gene induction by cAMP protects macrophages towards apoptosis that occurs as a result of excessive NO formation or addition of chemotherapeutica. Attenuating programmed cell death by the cAMP-signaling system may be found in association with Cox-2 expression and tumor formation.
Similar content being viewed by others
References
Eastman A, Rigas JR: Modulation of apoptosis signaling pathways and cell cycle regulation. Semin Oncol 26: 7–16, 1999
Hinds PW, Weinberg RA: Tumor suppressor genes. Curr Opin Genet Dev 4: 135–141, 1994
Hale AJ, Smith CA, Sutherland LC, Stoneman VEA, Longthorne VL, Culhane AC et al.: Apoptosis: Molecular regulation of cell death. Eur J Biochem 236: 1–26, 1996
Lee JM, Bernstein A: Apoptosis, cancer and the p53 tumour suppressor gene. Cancer Metastasis Rev 14: 149–161, 1995
Ridgway WM, Weiner HL, Fathman CG: Regulation of autoimmune response. Curr Opin Immunol 6: 946–955, 1994
Fadeel B, Orrenius S, Zhivotovsky B: Apoptosis in human disease: A new skin for the old ceremony? Biochem Biophys Res Commun 266: 699–717, 1999
Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH: Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84: 1415–1420, 1994
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM et al.: Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182: 1545–1556, 1995
Nicholson DW: Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Diff 6: 1028–1042, 1999
Petit PX, O'Connor JE, Grunwald D, Brown SC: Analysis of the membrane potential of rat-and mouse-liver mitochondria by flow cytometry and possible applications. Eur J Biochem 194: 389–397, 1990
Okada S, Zhang H, Hatano M, Tokuhisa T: A physiologic role of BclxL induced in activated macrophages. J Immunol 160: 2590–2596, 1998
Brockhaus F, Brü ne B: U937 apoptotic cell death by nitric oxide: Bcl-2 downregulation and caspase activation. Exp Cell Res 238: 33–41, 1998
May P, May E: Twenty years of p53 research: Structural and functional aspects of the p53 protein. Oncogene 18: 7621–7636, 1999
Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR: The p53 network. J Biol Chem 273: 1–4, 1998
Levine AJ: p53, the cellular gatekeeper for growth and division. Cell 88: 323–331, 1997
Watson RW, Redmond HP, Bouchier-Hayes D: Role of endotoxin in mononuclear phagocyte-mediated inflammatory responses. J Leukoc Biol 56: 95–103, 1994
Lander HM: An essential role for free radicals and derived species in signal transduction. FASEB J 11: 118–124, 1997
James PE, Grinberg OY, Swartz HM: Superoxide production by phagocytosing macrophages in relation to the intracellular distribution of oxygen. J Leukoc Biol 64: 78–84, 1998
Herschman HR, Reddy ST, Xie W: Function and regulation of prostaglandin synthase-2. Adv Exp Med Biol 407: 61–66, 1997
Hempel SL, Monick MM, He B, Yano T, Hunninghake GW: Synthesis of prostaglandin H synthase-2 by human alveolar macrophages in response to lipopolysaccharide is inhibited by decreased cell oxidant tone. J Biol Chem 269: 32979–32984, 1994
Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H et al.: Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem 267: 25934–25938, 1992
O'Sullivan MG, Huggins EMJ, Meade EA, DeWitt DL, McCall CE: Lipopolysaccharide induces prostaglandin H synthase-2 in alveolar macrophages. Biochem Biophys Res Commun 187: 1123–1127, 1992
Williams CS, Mann M, DuBois RN: The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 18: 7908–7916, 1999
Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA: Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem 274: 22903–22906, 1999
Smith WL, Garavito RM, DeWitt DL: Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and-2. J Biol Chem 271: 33157–33160, 1996
Coleman RA, Smith WL, Narumiya S: International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46: 205–229, 1994
Brü ne B, von Knethen A, Sandau KB: Nitric oxide (NO): An effector of apoptosis. Cell Death Diff 6: 969–975, 1999
von Knethen A, Brü ne B: Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J 11: 887–895, 1997
von Knethen A, Callsen D, Brü ne B: NF-kappaB and AP-1 activation by nitric oxide attenuated apoptotic cell death in RAW 264.7 macrophages. Mol Biol Cell 10: 361–372, 1999
von Knethen A, Callsen D, Brü ne B: Superoxide attenuates macrophage apoptosis by NF-kappaB and AP-1 activation that promotes cyclooxygenase-2 expression. J Immunol 163: 2858–2866, 1999
von Knethen A, Brockhaus F, Kleiter I, Brü ne B: NO-Evoked macrophage apoptosis is attenuated by cAMP-induced gene expression. Mol Med 5: 672–684, 1999
von Knethen A, Lotero A, Brü ne B: Etoposide and cisplatin induced apoptosis in activated RAW 264.7 macrophages is attenuated by cAMPinduced gene expression. Oncogene 17: 387–394, 1998
McConkey DJ, Nicotera P, Hartzell P, Bellomo G, Wyllie AH, Orrenius S: Glucorticoids activate a suicide process in thymocytes through an elevation of cytosolic Ca2+ concentration. Arch Biochem Biophys 269: 365–370, 1989
Burton K: A study of the conditions and mechanisms of the diphenylamine reaction for the estimation of deoxyribonucleic acid. Biochem J 62: 315–323, 1956
Schoonbroodt S, Legrand Poels S, Best Belpomme M, Piette J: Activation of the NF-kappaB transcription factor in a T-lymphocytic cell line by hypochlorous acid. Biochem J 321: 777–785, 1997
Camandola S, Leonarduzzi G, Musso T, Varesio L, Carini R, Scavazza A et al.: Nuclear factor kappa B is activated by arachidonic acid but not by eicosapentaenoic acid. Biochem Biophys Res Commun 229: 643–647, 1996
Sassone-Corsi P, Visvader J, Ferland L, Mellon PL, Verma IM: Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev 2: 1529–1538, 1988
Hart TW: Some observations concerning the S-nitroso and S-phenylsulphonyl derivates of L-cysteine and glutathione. Tetrahedron Lett 26: 2013–2016, 1985
Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN: Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 107: 1183–1188, 1994
Kargman SL, O'Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S: Expression of prostaglandin G/H synthase-1 and-2 protein in human colon cancer. Cancer Res 55: 2556–2559, 1995
Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K et al.: Expression of cyclooxygenase-1 and-2 in human colorectal cancer. Cancer Res 55: 3785–3789, 1995
Levy GN: Prostaglandin H synthases, nonsteroidal antiinflammatory drugs, and colon cancer. FASEB J 11: 234–247, 1997
Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E et al.: Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87: 803–809, 1996
Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J et al.: Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 99: 2254–2259, 1997
Lo CJ, Fu M, Lo FR, Cryer HG: Cyclooxygenase 2 (COX-2) gene activation is regulated by cyclic adenosine monophosphate. Shock 13: 41–45, 2000
Zhang F, Warskulat U, Wettstein M, Schreiber R, Henninger HP, Decker K et al.: Hyperosmolarity stimulates prostaglandin synthesis and cyclooxygenase-2 expression in activated rat liver macrophages. Biochem J 312: 135–143, 1995
Vial D, Arbibe L, Havet N, Dumarey C, Vargaftig B, Touqui L: Downregulation by prostaglandins of type-II phospholipase A2 expression in guinea-pig alveolar macrophages: A possible involvement of cAMP. Biochem J 330: 89–94, 1998
Kawase T, Orikasa M, Suzuki A: Phorbol ester-like action of staurosporine on the cAMP response to prostaglandin E2 in two macrophagelike cell lines at distinct differentiation stages. Cell Signal 4: 479–485, 1992
Fladmark KE, Gjertsen BT, Doskeland SO, Vintermyr OK: Fas/APO-1(CD95)-induced apoptosis of primary hepatocytes is inhibited by cAMP. Biochem Biophys Res Commun 232: 20–25, 1997
Parvathenani LK, Buescher ES, Chacon-Cruz E, Beebe SJ: Type I cAMP-dependent protein kinase delays apoptosis in human neutrophils at a site upstream of caspase-3. J Biol Chem 273: 6736–6743, 1998
Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD: Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 286: 2358–2361, 1999
Torgersen KM, Vaage JT, Levy FO, Hansson V, Rolstad B, Tasken K: Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem 272: 5495–5500, 1997
Walker BA, Rocchini C, Boone RH, Ip S, Jacobson MA: Adenosine A2a receptor activation delays apoptosis in human neutrophils. J Immunol 158: 2926–2931, 1997
Stefanelli C, Stanic I, Bonavita F, Flamigni F, Pignatti C, Guarnieri C et al.: Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem Biophys Res Commun 243: 821–826, 1998
Nakajima T, Uchida C, Anderson SF, Parvin JD, Montminy M: Analysis of a cAMP-responsive activator reveals a two-component mechansim for transcriptional induction via signal-dependent factors. Genes Dev 11: 738–747, 1997
Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M et al.: Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature 370: 226–229, 1994
Scheid MP, Foltz IN, Young PR, Schrader JW, Duronio V: Ceramide and cyclic adenosine monophosphate (cAMP) induce cAMP response element binding protein phosphorylation via distinct signaling pathways while having opposite effects on myeloid cell survival. Blood 93: 217–225, 1999
Yang YM, Dolan LR, Ronai Z: Expression of dominant negative CREB reduces resistance to radiation of human melanoma cells. Oncogene 12: 2223–2233, 1996
Messmer UK, Reed UK, Brü ne B: Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J Biol Chem 271: 20192–20197, 1996
Brockhaus F, Brü ne B: p53 accumulation in apoptotic macrophages is an energy demanding process that precedes cytochrome c release in response to nitric oxide. Oncogene 18: 6403–6410, 1999
Du K, Montminy M: CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273: 32377–32379, 1998
Jean D, Harbison M, McConkey DJ, Ronai Z, Bar-Eli M: CREB and its associated proteins act as survival factors for human melanoma cells. J Biol Chem 273: 24884–24890, 1998