Abstract
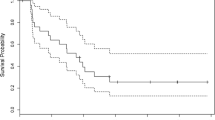
This study included 39 patients (37 evaluable, of whom 30 patients with recurrent gliomas and 7 patients with gliomas untreated by radiotherapy); they were enrolled into a phase II trial using a new nitrosourea, cystemustine, administrated every 2 weeks at 60 mg/m2 as a 15 min-infusion. Pathology at inclusion was (WHO classification): 14 glioblastomas, 20 grade 3–4 astrocytomas and 3 grade 3 oligodendrogliomas. Four partial responses have been obtained, giving an overall response rate of 10.8%. Four additional patients had a partial response, which for various reasons was not confirmed 4 weeks later; 12 patients had a stable disease for at least 8 weeks, 15 patients had progressive disease. Of the 4 responses, 2 were with a grade 3 oligodendroglioma and 2 glioblastoma. Toxicity (WHO grading) was mainly hematological: leukopenia (16.2% grade 3–4), neutropenia (29.7% grade 3–4), thrombopenia (27% grade 3–4). No other toxicity greater than grade 2 was observed. In conclusion, cystemustine at 60 mg/m2 has moderate clinical activity in relapsing glioma. Our results warrant further investigation of this agent with an increased dose or modified scheme.
Similar content being viewed by others
References
Malkin MG, Shapiro WR: Brain tumors. Cancer Chemother Biol Response Modif: 377-389, 1990
Walker MD, Green SB, Byar DP et al.: Randomized comparison of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Eng J Med 303: 1323-1329, 1980
Fine HA, Dear KBG, Loeffler Js et al.: Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 71: 2585-2597, 1993
Feun LG, Savaraj N, Landy HJ: Drug resistance in brain tumors. J Neurooncol 20(2): 165-176, 1994
Kirby S, Brothers M, Irish W et al.: Evaluating glioma therapies: modeling treatments and predicting outcomes. J Natl Cancer Inst 87(24): 1884-1888, 1995
Madelmont JC, Godenèche D, Parry D et al.: New cysteamin (2-chloroethyl) nitrosoureas. Synthesis and preliminary antitumor results. J Med Chem 29(9): 1346-1350, 1985
Godenèche D, Madelmont JC, Moreau MF et al.: Metabolic disposition of 2-chloroethyl nitrosocarbamylcystamine in rats. Drug Métab and Dispos 13: 220-226, 1985
Bourrut C, Chenu E, Godenèche D: Cytostatic action of two nitrosoureas derived from cysteamine. Br J Pharmac 89: 539-546, 1986
MacDonald DR, Cascino TL, Schold SCJ et al.: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277-1280, 1990
Kornblith PL, Walker M: Chemotherapy for malignant gliomas. J Neurosurg 68: 1-17, 1988
De Angelis LM, Burger PC, Green SB et al.: Adjuvant chemotherapy for malignant glioma: who benefits? Ann Neurol 40: 491-492, 1996
Mason W, Louis D, Cairncross J: Chemosensitive gliomas in adults: which ones and why? J Clin Oncol 15: 3423-3426, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roche, H., Cure, H., Adenis, A. et al. Phase II Trial of Cystemustine, a New Nitrosourea, as Treatment of High-grade Brain Tumors in Adults. J Neurooncol 49, 141–145 (2000). https://doi.org/10.1023/A:1026524825573
Issue Date:
DOI: https://doi.org/10.1023/A:1026524825573