Abstract
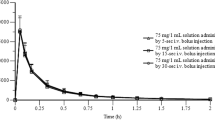
Pharmacokinetics of diltiazem and its six metabolites were compared after oral administration in dogs of a multiparticulate sustained-release diltiazem preparation (HER-SR, QD) and a conventional diltiazem preparation (HER, TID). The plasma concentration of diltiazem, its two active basic metabolites (Ml, N-monodesmethyl diltiazem; M2, deacetyl diltiazem), and four acidic metabolites [Al, ( + )-(2S,3S)-2-(4-methoxyphenyl)-3-acetoxy-4-oxo-2,3,4,5,-tetrahydro-l,5-benzothiazepin-5-acetic acid; A2, 3-deacetyl-Al; A3, O-demethyl-Al; A4, O-demethyl-3-deacetyl-Al] following several administration routes were determined using high-performance liquid chromatography with UV detector (UV-HPLC). Following the oral administration of HER to dogs, plasma concentrations were in the descending order of A2, diltiazem, Ml, and M2. The absolute bioavailability of diltiazem was about 30%. Diltiazem conversion to its metabolites (Ml, M2, A2) was 31.0, 2.1, and 14.6%, respectively. Following intraduodenal and mesenteric venous administration of diltiazem, Ml and A2 were produced mainly in the intestine and liver. Oral administration of HER-SR and HER to dogs resulted in almost-identical plasma concentrations of A2, diltiazem, Ml, and M2 (descending order). Supported evidence was the effective absorption of diltiazem from all gastrointestinal tract regions and similar formation ratios of diltiazem basic metabolites (Ml, M2) from the duodenum, ileum, and colon.
Similar content being viewed by others
REFERENCES
M. Sato, T. Nagao, I. Yamaguchi, H. Nakajima and A. Kiyomoto. Pharmacological studies on a new 1,5-benzothiazepine derivative (CRD-401). Arzneim. Forsch. 21:1338–1343 (1971).
K. Murata, H. Yamahara, M. Kobayashi, K. Noda, and M. Samejima. Pharmacokinetics of an oral sustained-release diltiazem preparation. J. Pharm. Sci. 78:960–963 (1989).
J. Sugihara, Y. Sugawara, H. Ando, S. Harigaya, A. Etoh, and K. Kohno. Studies on the metabolism of diltiazem in man. J. Pharm. Dyn. 7:24–32 (1984).
Y. Sugawara, M. Ohashi, S. Nakamura, S. Usuki, T. Suzuki, Y. Ito, T. Kume, S. Harigaya, A. Nakao, M. Gaino, and H. Inoue. Metabolism of diltiazem I. Structures of new acidic and basic metabolites in rat, dog and man. J. Pharmacobio-Dyn. 11:211–223 (1988).
M. Okada, H. Inoue, Y. Nakamura, M. Kishimoto, and T. Suzuki. Excretion of diltiazem in human milk. Yakuri Rinsyou 13:1609–1612 (1985).
T. Meshi, J. Sugihara, and Y. Sato. Metabolic fate of d-cis-3-acetoxy-5-(2-(dimethylamino)ethyl)-2,3-dihydro-2-(p-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one hydrochloride(CRD-401). Chem. Pharm. Bull. 19:1546–1556 (1971).
K. Murata and K. Noda. Pharmacokinetic analysis of an oral sustained-release diltiazem preparation using multifraction absorption models. Pharm. Res. 10:757–762 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murata, K., Yamahara, H. & Noda, K. Pharmacokinetics of Diltiazem and Its Metabolites in Dogs After Oral Administration of a Multiparticulate Sustained-Release Preparation. Pharm Res 10, 1165–1168 (1993). https://doi.org/10.1023/A:1018916201735
Issue Date:
DOI: https://doi.org/10.1023/A:1018916201735