Abstract
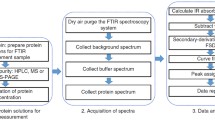
Second derivative infrared (IR) spectroscopy can be used as a quick, easy, reproducible, cost-effective, non-destructive tool by which to evaluate the purity and structural integrity of samples of water-soluble proteins from a variety of sources. For this study, second derivative IR spectra were collected at ambient conditions for aqueous (D2O) solutions of seven different commercial samples of the same enzyme, porcine pancreatic elastase (2.0 to 3.8 mg protein/100 µL D2O, pD = 5.4 to 9.1). As with other globular proteins possessing a large fraction of β-structure, the amide I′ region [1700-1620 cm−1] of the second derivative IR spectra for each of the seven elastase samples exhibits a characteristic pair of bands: one of weak intensity appears near 1684 cm−1; the other close to 1633 cm−1is moderate-to-strong. However, one of the seven samples shows a striking decrease in the observed intensities of the amide I′ bands relative to the 1516 cm−1 absorption, along with the appearance of a strong, new band at 1614 cm−1. These intensity disparities strongly suggest that this sample is of much lower quality than the others and clearly has an appreciable proportion of the protein present in a non-native state. In addition, minor differences evident in the position and relative intensity of some individual amide I′ bands among the seven spectra imply that subtle variations exist in the conformation of the peptide backbone of the seven samples. For two of the samples, these small, but reproducible, changes seem to be correlated with marked losses of enzyme activity. Finally, bands outside the amide I′ region may prove useful in assessing sample purity and identifying non-protein contaminants.
Similar content being viewed by others
REFERENCES
W. Surewicz, H. H. Mantsch, and D. Chapman. Determination of protein secondary structure by Fourier transform infrared spectroscopy: A critical assessment. Biochemistry 32:389–394 (1993).
J. Bandekar. Amide modes and protein conformation. Biochim. Biophys. Acta 1120:123–143 (1992).
W. Surewicz and H. H. Mantsch. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim. Biophys. Acta 942:115–130 (1988).
H. Susi and D. M. Byler. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Meth. Enzymol. 130:290–311 (1986).
R. K. Scopes. Protein Purification: Principles and Practice. Springer-Verlag, New York, 1982, pp. 213–259
H. Susi and D. M. Byler. Protein structure by Fourier transform infrared spectroscopy: Second derivative spectra. Biophys. Biochem. Res. Commun. 115:391–397 (1983).
R. J. Markovich and C. Pidgeon. Introduction to Fourier transform infrared spectroscopy and applications in the pharmaceutical sciences. Pharm. Res. 8:663–675 (1991).
S. J. Prestrelski, T. Arakawa, and J. F. Carpenter. Separation of freezing-and drying-induced denaturation of lyophilized proteins using stress-specific stabilization: II. Structural studies using infrared spectroscopy. Arch. Biochem. Biophys. 303:465–473 (1994).
J. G. Bieth. Elastases: Catalytic and biological properties. In R. P. Mecham (ed.), Biology of Extracellular Matrix: Regulation of Matrix Accumulation, Academic Press, New York, 1986, pp. 217–320.
W. Bode, E. Meyer, Jr., and J. C. Powers. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry 28:1951–1963 (1989).
M. Levitt and J. Greer. Automatic identification of secondary structure of globular proteins. J. Mol. Biol. 114:181–239 (1977).
S. J. Prestrelski, D. M. Byler, and M. N. Liebman. Generation of a substructure library for the description and classification of protein secondary structure. II. Application to spectra-structure correlations in Fourier transform infrared spectroscopy. Proteins: Struct. Func. Gen. 14:440–450 (1992).
G. Zuber, S. J. Prestrelski, and K. Benedek. Application of Fourier transform infrared spectroscopy to studies of aqueous protein solutions. Anal. Biochem. 207:150–156 (1992).
E. G. Bendit. Infrared absorption of tyrosine side chains in proteins. Biopolymers 5:525–533 (1967).
U. K. Laemmli. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond) 227:680–687 (1970).
Worthington, C. C., ed. Worthington Biochemical Manual. Worthington Biochemical Corp., Freehold, NJ, 1988. p. 141.
G. Feinstein, A. Kupfer, and M. Sokolovsky. N-Acetyl-(L-ala)3-p-nitroanilide as a new chromogenic substrate for elastase. Biochem. Biophys. Res. Commun. 50:1020 (1973).
M. M. Bradford. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254.
M. Jackson and H. H. Mantsch. Halogenated alcohols as solvents for proteins: FTIR spectroscopic studies. Biochim. Biophys. Acta 1118:139–143 (1991).
D. Carrier, H. H. Mantsch, and P. T. T. Wong. Pressure-induced reversible changes in secondary structure of poly(L-lysine): An IR spectroscopic study. Biopolymers 29:837–844 (1990).
D. M. Byler and J. M. Purcell. FTIR examination of thermal denaturation and gel-formation in whey proteins. Proc. Soc. Photo Opt. Instrum. Eng. (Fourier Transform Spectroscopy) 1145:415–417 (1989).
K. Ito and H. J. Bernstein. Vibrational spectra of the formate, acetate, and oxalate ions. Can. J. Chem. 34:170–178 (1956).
N. B. Colthup, L. H. Daly, and S. E. Wiberley. Introduction to Infrared and Raman Spectroscopy, Academic Press, Boston, 3rd ed., 1990.
D. C. Lee and D. Chapman. Infrared spectroscopic studies of biomembranes and model membranes. Biosci. Rept., 6:235–256 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Byler, D.M., Wilson, R.M., Randall, C.S. et al. Second Derivative Infrared Spectroscopy as a Non-Destructive Tool to Assess the Purity and Structural Integrity of Proteins. Pharm Res 12, 446–450 (1995). https://doi.org/10.1023/A:1016273122944
Issue Date:
DOI: https://doi.org/10.1023/A:1016273122944