Abstract
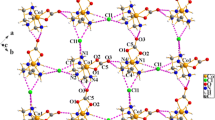
A room temperature water solution of (I) crystallizes as a racemate, space groupP2 1/n with lattice constantsa=7.737(6),b=10.694(5),c=15.097(6) Å, andβ=102.83(5)°;V=1218.05 Å3 andd (calc; M.W.=337.24, Z=4) = 1.642 g cm−3. A total of 2381 data were collected over the range 4° ≤ 2θ < 50°; of these, 1452 (independent and withI ≥ 3σ(I)) were used in the structural analysis. Data were corrected for absorption (μ = 15.76 cm−1), and the relative transmission coefficients ranged from 0.8976 to 0.9984. Refinement led to the finalR(F) andR w(F) residuals of 0.0858 and 0.1116. A room temperature water solution of (II) crystallizes as a racemate in space group P21/c with lattice constantsa=6.638(3),b=11.425(8),c=15.147(16) Å, andβ=93.27(6)°; F=1146.8 Å andd (calc; M.W.=323.2,Z=4) = 1.872 g cm−3. A total of 2200 data were collected over the range 4° ≤ 2θ < 50°; of these, 1918 (independent and withI ≥ 3σ(I)) were used in the structural analysis. Data were corrected for absorption (μ=16.94 cm−1), and the relative transmission coefficients ranged from 0.9049 to 0.9967. Refinement led to the finalR(F) andR w(F) residuals of 0.0231 and 0.0279. The chirality symbol for the particular enantiomer of (I) refined here is ∧ (δδ), while for (II) the chirality symbol is ∧(δλ), which means that in the latter compound one of the en rings is in a higher energy conformation. We attribute this result to competitive intramolecular hydrogen-bonded interactions between the — NH2 hydrogens of the en ligands and the oxygens of the -NO2 and -SO3 ligands, strengths which are enhanced by coercing a change in sign of the torsional angle of one en ringa motion which permits both oxo ligands to form stronger hydrogen bonds while retaining proper O ⋯ O contacts. This phenomenon is not observed in (I) since the azide ligand does not compete with -SO3 for such hydrogen-bonded interactions, and nonbonded pair repulsions can be minimized without affecting the ability of — SO3 oxygens to form strong intramolecular hydrogen bonds.
Similar content being viewed by others
References
Bernal, I.Inorg. Chim. Acta 1985,96, 99.
Bernal, I.Inorg. Chim. Acta 1985,101, 175.
Bernal, I.Inorg. Chim. Acta 1988,142, 21.
Bernal, I.; Cetrullo, J.Inorg. Chim. Acta 1987,144, 227.
Bernal, I.; Cetrullo, J.Inorg. Chim. Acta 1987,150, 75.
Bernal, I.; Cetrullo, J.J. Coord. Chem. 1989,20, 259.
Bernal, I.; Cetrullo, J.Struct. Chem. 1990,1, 227.
Bernal, I.; Cetrullo, J.Struct. Chem. 1990,1, 235.
Bernal, I.; Cetrullo, J.; Berhane, S.Struct. Chem. 1990,1, 361.
Ralston, C. L.; White, A. H.; Yandell, J. K.Aust. J. Chem. 1979,32, 291.
Ralston, C. L.; White, A. H., Yandell, J. K.Aust. J. Chem. 1978,31, 999.
Jackson, W. G.Inorg. Chem. 1988,27, 111.
Analyses by Galbraith Laboratories, Inc., 2323 Sycamore Dr., Knoxville, TN 37921–1750.
Buckingham, D. A.; Cresswell, P. J.; Jackson, W. G.; Sargeson, A. M.Inorg. Chem. 1981,20, 1647.
Molecular Structure Corporation, 3200 Research Forest Dr., The Woodlands, Texas 77386.
B. A. Frenz & Associates, 1140 East Harvey Road, College Station, Texas 77840.
Roof, R. B.A Theoretical Extension of the Reduced Cell Concept in Crystallography, Report LA-4038, Los Alamos Scientific Laboratory, 1969.
Cromer, D. T.; Waber, J. T.International Tables for X-ray Crystallography; Kynoch Press: Birmingham, England, 1975; Vol. IV, Tables 2.2.8 and 2.3.1, respectively, for the scattering factor curves and the anomalous dispersion values.
Kastner, M.; Smith, D. A.; Kuzmission, A. G.; Cooper, J. N.; Tyree, T.; Yearick, M.Inorg. Chim. Acta 1989,158, 185.
Kuroda, Y.; Tanaka, N.; Goto, M.; Sakai, T.Inorg. Chem. 1989,28, 997.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bernal, I., Cetrullo, J. & Jackson, W.G. The phenomenon of conglomerate crystallization in coordination compounds. XXIII: The crystallization behavior of [cis-Co(en)2(N3)(SO3)] · 2H2O (I) and of [cis-Co(en)2(NO2)(SO3)] · H2O (II). Struct Chem 4, 235–245 (1993). https://doi.org/10.1007/BF00673698
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00673698