Abstract
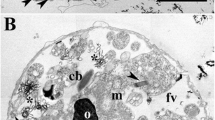
The morphology and ultrastructure of the thermophilic cyanobacteriumMastigocladus laminosus were examined by scanning and transmission electron microscopy. Mature cultures consisted of relatively old, wide filaments that branched frequently to form younger, thinner filaments. The cells of the younger filaments had a consistently cylindrical morphology, while those of older filaments were rounded and pleomorphic. The internal ultrastructure of the cells depended somewhat on their age. As young cells became larger and wider, their thylakoids underwent slight rearrangement and spread out toward the center of the cytoplasm. Polyphosphate bodies, carboxysomes (polyhedral bodies), and lipid-body-like structures increased in number as the cells aged, but ribosomes and cyanophycin granules were depleted. Cell division involved septum formation followed by ingrowth of the outer membrane and sheath. Cells in older filaments were separated from each other by a complete layer of sheath material. Septum formation in older cells was also seen to occur parallel to the long axis of the filament, thereby confirming that true branching took place.
Similar content being viewed by others
References
Allen MM (1972) Mesosomes in blue-green algae. Arch Microbiol 84:199–206
Allen MM, Weathers PJ (1980) Structure and composition of cyanophycin granules in the cyanobacteriumAphanocapsa 6308. J. Bacteriol 141:959–962
Avakyan AA, Kats LN, Mineeva LA, Rathner EN, Rathner EN, Gusev MV (1978) Electron microscopic data on mesosome-like and myelin-like structures in blue-green algae. Microbiologya Transl 47:739–744
Blwill DL, Maratea D, Blakemore RP (1980) Ultrastructure of a magnetotactic bacterium. J Bacteriol 141:1399–1408
Balkwill DL, Stevens SE, Jr. (1980) Effects of penicillin G on mesosome-like structures inAgmenellum quadruplicatum. Antimicrob Agents Chemother 17:506–509
Burdett IDJ, Murray RGE (1974a) Electron microscope study of septum formation inEscherichia coli strains B and B/r during synchronous growth. J Bacteriol 119:1039–1056
Burdett IDJ, Murray RGE (1974b) Septum formation inEscherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol 119:303–324
Butler RD, Allsopp A (1972) Ultrastructural investigations in the Stigonemataceae (Cyanophyta). Arch Mikrobiol 82:283–299
Castenholz RW (1976) The effect of sulfide on the blue-green algae of hot springs. I. New Zealand and Iceland. J Phycol 12:54–68
Castenholz RW (1977) The effect of sulfide on the blue-green algae of hot springs. II. Yellowstone National Park. Micrb Ecol 3:79–105
Costerton JW, Ingram JM, Cheng K-J (1974) Structure and function of the cell envelope of Gram-negative bacteria. Bacteriol Rev 38:87–110
Drews G (1973) Fine structure and chemical composition of the cell envelopes. In: Carr NG, Whitton BA (eds) The biology of blue-green algae. University of California Press, Los Angeles, pp 96–116
Echlin P (1964) Intra-cytoplasmic membranous inclusions in the blue-green alga,Anacystis nidulans. Arch Mikrobiol 49:267–274
Edwards MR, Berns DS, Ghiorse WC, Holt SC (1968) Ultrastructure of the thermophilic blue-green alga,Synechococcus lividus Copeland. J Phycol 4: 283–298
Edwards MR, Gantt E (1971) Phycobilisomes of the thermophilic blue-green alga,Synechococcus lividus. J Cell Biol 50:898–900
Eykelenburg C Van (1979) The ultrastructure ofSpirulina platensis in relation to temperature and light intensity. Antonie van Leeuwenhoek 45:369–390
Eykelenburg C Van (1980) Ecophysiological studies onSpirulina platensis. Effect of temperature, light intensity and nitrate concentration on growth and ultrastructure. Antonie van Leeuwenhoek 46:113–127
Fagerberg WR, Arnott HJ (1979) Seasonal changes in structure of a submerged blue-green algal/bacterial community from a geothermal hotspring. J Phycol 15:445–452
Fritsch FE (1954) The structure and reproduction of algae. Cambridge Univ. Press, New York
Gantt E, Conti SF (1969) Ultrastructure of blue-green algae. J Bacteriol 97:1486–1493
Gilleland HE, Jr., Murray RGE (1975) Demonstration of cell division by septation in a variety of Gram-negative rods. J Bacteriol 121:721–725
Ingram LO, Thurston EL (1970) Cell division in morphological mutants ofAgmenellum quadruplicatum, strain BG-1. Protoplasma 71:55–75
Jensen TE (1968) Electron microscopy of polyphosphate bodies in a blue-green alga,Nostoc pruniforme. Arch Mikrobiol 62:144–152
Jensen TE (1969) Fine structure of developing polyphosphate bodies in a blue-green alga,Plectonema boryanum. Arch Mikrobiol 67:328–338
Jensen TE, Sicko-Goad L, Ayala RP (1977) Phosphate metabolism in blue-green algae. III. The effect of fixation and poststaining on the morphology of polyphosphate bodies inPlectonema boryanum. Cytologia 42:357–369
Kellenberger E, Ryter A, Sechaud J (1958) Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol 4:671–687
Khan ZNT, Godward MBE (1978) Cyanophycin granules in a blue-green algaCalothrix marchica Lemm. Cur Sci 47:710–712
Lang NJ, Fisher KA (1969) Variation in the fixation image of “structured granules” inAnabaena. Arch Mikrobiol 67:173–181
Lang NJ, Simon RD, Wolk CP (1972) Correspondence of cyanophycin granules with structured granules inAnabaena cylindrica. Arch Mikrobiol 83:313–320
Lee S (1927) Cytological study ofStigonema mammilosum. Bot Gaz 83:420–424
Marcenko E (1961) Licht- und elektronenmikroskopische Untersuchungen an der ThermalalgeMastigocladus laminosus Cohn. Acta Bot Croatica 20/21:47–74
Martin TC, Wyatt JT (1974) Comparative physiology and morphology of six strains of stigonematacean blue-green algae. J Phycol 10:57–65
Miura Y, Yokoyama H, Kanaoka K, Saito S, Iwasa K, Okazaki M, Komemushi S (1980) Hydrogen evolution by a thermophilic blue-green algaMastigocladus laminosus. Plant Cell Physiol 21:149–156
Miyamoto K, Hallenbeck PC, Benemann JR (1979a) Nitrogen fixation by thermophilic blue-green algae (cyanobacteria): temperature characteristics and potential use in biophotolysis. Appl Environ Microbiol 37:454–458
Miyamoto K, Hallenbeck PC, Benemann JR (1979b) Hydrogen production by the thermophilic algaMastigocladus laminosus: effects of nitrogen, temperature, and inhibition of photosynthesis. Appl Environ Microbiol 38:440–446
Reynolds ES (1963) The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J Cell Biol 17:208–212
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria J Gen Microbiol 111:1–61
Ris H, Singh RN (1961) Electron microscope studies on blue-green algae. J Biophys Biochem Cytol 9:63–80
Schwabe GH (1960) Über den thermobionten KosmopolitenMastigocladus laminosus Cohn. Hydrol 22:759–792
Spearing JK (1937) Cytological studies of the Myxophyceae. Arch Protistenk 89:209–278
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Stanier RY, Cohen-Bazire G (1977) Phototrophic prokaryotes: the cyanobacteria. Ann Rev Microbiol 31:225–274
Stevens SE, Jr., Balkwill DL, Paone DAM (1981a) The effect of nitrogen limitation on the ultrastructure of the cyanobacteriumAgmenellum quadruplicatum. Arch Microbiol 130:204–212
Stevens SE, Jr., Paone DAM, Balkwill DL (1981b) Accumulation of cyanophycin granules as a result of phosphate limitation inAgmenellum quadruplicatum. Plant Physiol 67:716–719
Stevens SE, Jr., Patterson COP, Myers J (1973) The production of hydrogen peroxide by blue-green algae: a survey. J Phycol 9:427–430
Thurston EL, Ingram LO (1971) Morphology and fine structure ofFischerella ambigua. J Phycol 7:203–210
Wolk CP (1973) Physiology and cytological chemistry of blue-green algae. Bacteriol Rev 37:32–101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nierzwicki, S.A., Maratea, D., Balkwill, D.L. et al. Ultrastructure of the cyanobacterium,Mastigocladus laminosus . Arch. Microbiol. 133, 11–19 (1982). https://doi.org/10.1007/BF00943762
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00943762