Abstract
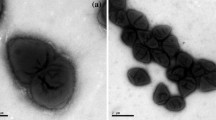
A strictly anaerobic, uric acid, xanthine, and guanine fermenting bacterium was isolated from sewage sludge requiring thiamine as a vitamin. Acetate, formate, ammonia and CO2 were products. Cells were Gram-positive, straight rods, 3 to 6.5 μm long and 1.1 to 1.5 μm wide. They were non-motile, however, possessed flagella. Spore formation could not be obtained. The guanine-plus-cytosine content (G+C) of its deoxyribonucleic acid was 40.3 mol%. Based on these features, the organism belongs to the genus Eubacterium. Due to its restricted substrate spectrum and its inability to utilize arginine or to form cytochromes like E. lentum, this organism did not resemble any of the previously described species of Eubacterium. Therefore, it is proposed to form a new species Eubacterium angustum sp. nov.
Similar content being viewed by others
References
Bahl H, Andersch W, Gottschalk G (1982) Continuous production of acetone and butanol by Clostridium acetobutylicum in a two-stage phosphate limited chemostat. Eur J Appl Microbiol Biotechnol 15:201–205
Barker HA (1961) Fermentations of nitrogenous organic compounds. In: Gunsalus IC, Stanier RY (eds) The bacteria, vol 2. Academic Press, New York, pp 151–207
Barker HA, Beck JV (1941) The fermentative decomposition of purines by Clostridium acidiurici and Clostridium cylindrosporum. J Biol Chem 141:3–27
Barker HA, Beck JV (1942) Clostridium acidiurici and Clostridium cylindrosporum, organisms fermenting uric acid and some other purines. J Bacteriol 43:291–304
Barnes EM, Impey CS (1974) The occurrence and properties of uric acid decomposing anaerobic bacteria in the avian caecum. J Appl Bacteriol 37:393–409
Buchanan RE, Gibbons NE (1974) Bergey's manual of determinative bacteriology, 8th edn. The Williams & Wilkins Co, Baltimore
Braun M, Schoberth S, Gottschalk G (1979) Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol 120:201–204
Champion AB, Rabinowitz JC (1977) Ferredoxin and formyltetrahydrofolate synthetase: comparative studies with Clostridium acidiurici, Clostridium cylindrosporum, and newly isolated anaerobic uric acid-fermenting strains. J Bacteriol 132:1003–1020
DeLey J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Dorn M, Andreesen JR, Gottschalk G (1978) Fermentation of fumarate and L-malate by Clostridium formicoaceticum. J Bacteriol 133:26–32
Dürre P, Andreesen JR (1982) Separation and quantitation of purines and their anaerobic and aerobic degradation products by high-pressure liquid chromatography. Anal Biochem 123:32–40
Dürre P, Andreesen JR (1983) Purine and glycine metabolism by purinolytic clostridia. J Bacteriol 154:192–199
Dürre P, Andersch W, Andreesen JR (1981) Isolation and characterization of an adenine utilizing, anaerobic sporeformer, Clostridium purinolyticum nov. spec. Int J Syst Bacteriol 31:184–194
Freese E, Heinze J, Mitani T, Freese EB (1978) Limitation of nucleotides induces sporulation. In: Chambliss G, Vary C (eds) Spores VII. American Society for Microbiology, Washington, pp 277–285
Holdemann LV, Moore WEC (1974) Genus Eubacterium. In: Buchanan RE, Gibbons NE (eds) Bergey's manual of determinative bacteriology, 8th edn. The Williams & Wilkins Co. Baltimore, pp 641–657
Holdemann LV, Cato EP, Moore WEC (1977) Anaerobe laboratory manual, 4th edn. Anaerobe Laboratory Virginia Polytechnic Institute and State University, Blacksburg
Holdemann LV, Cato PE, Burmeister JA, Moore WEC (1980) Descriptions of Eubacterium timidum sp. nov., Eubacterium brachy sp. nov., and Eubacterium nodatum sp. nov. isolated from human periodontitis. Int J Syst Bacteriol 30:163–169
Holländer R, Pohl S (1980) Deoxyribonucleic acid base composition of bacteria. Zbl Bakteriol Parasitenkd Infektionskr Hyg Abt I Orig A 246:236–275
Imhoff-Stuckle D, Kandler O, Mayer F, Spieß E, Andreesen JR Relationships of Clostridium barkeri to Eubacterium limosum and Acetobacterium woodii. System Appl Microbiol (in press)
Koransky JR, Allen SD, Dowell VR (1978) Use of ethanol for selective isolation of sporeforming microorganisms. Appl Environ Microbiol 35:762–765
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Mead GC, Adams BW (1975) Some observations on the caecal microflora of the chick during the first two weeks of life. Br Poult Sci 16:169–176
Reece P, Toth D, Dawes EA (1976) Fermentation of purines and their effect on the adenylate energy charge and viability of starved Peptococcus prévotii. J Gen Microbiol 97:63–71
Salanitro JP, Fairchilds IG, Zgornicki YD (1974) Isolation, culture characteristics, and identification of anaerobic bacteria from the chicken cecum. Appl Microbiol 27:678–687
Schäfer R, Schwartz AC (1976) Catabolism of purines in Clostridium sticklandii. Zbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig A 235:165–172
Schiefer-Ullrich H, Wagner R, Dürre P, Andreesen JR (1984) Comparative studies on physiology and taxonomy of obligately purinolytic clostridia. Arch Microbiol 138:344–353
Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36:407–477
Smith LDS, Hobbs G (1974) Genus Clostridium Prazmowski 1880, 23. In: Buchanan RE, Gibbons NE (eds) Bergey's manual of determinative bacteriology. 8th ed. The Williams & Wilkins Co, Baltimore, pp 551–572
Spahr R (1982) Anaerober Aminosäure- und Purinabbau durch einige Arten der Gattung Peptococcus. Diploma thesis, University of Göttingen
Sperry JF, Wilkins TD (1976) Arginine, a growth limiting factor for Eubacterium lentum. J Bacteriol 127:780–784
Stouthamer AH (1979) The search for correlation between theoretical and experimental growth yields. In: Quayle JR (ed) Microbial Biochemistry. University Park Press, Baltimore, pp 1–47
Tanner RS, Stackebrandt E, Fox GE, Woese CR (1981) A phylogenetic analysis of Acetobacterium woodii, Clostridium barkeri, Clostridium butyricum, Clostridium lituseburense, Eubacterium limosum, and Eubacterium tenue. Curr Microbiol 5:35–38
Tziaka C (1984) Anaerober Benzoatabbau durch Desulfococcus multivorans und Desulfosarcina variabilis. Diploma thesis, University of Göttingen
Vogels GD, van der Drift C (1976) Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev 40:403–468
Wagner R, Andreesen JR (1977) Differentiation between Clostridium acidiurici and Clostridium cylindrosporum on the basis of specific metal requirements for formate dehydrogenase fromation. Arch Microbiol 114:219–224
Walther-Mauruschat A, Aragno M, Mayer F, Schlegel HG (1977) Micromorphology of Gram-negative hydrogen bacteria. II. Cell envelope, membranes, and cytoplasmic inclusions. Arch Microbiol 114:101–110
Whiteley, HR (1952) The fermentation of purines by Micrococcus aerogenes. J Bacteriol 63:163–175
Whiteley HR, Douglas HC (1951) The fermentation of purines by Micrococcus lactilyticus. J Bacteriol 61:605–616
Wiegel J, Mayer F (1978) Isolation of lipopolysaccharide and the effect of polymyxin B on the outer membrane of Corynebacterium autotrophicum. Arch Microbiol 118:67–69
Wilber CG (1980) Toxicology of selenium: a review. Clin Toxicol 17:171–230
Author information
Authors and Affiliations
Additional information
This paper is dedicated to Prof. Dr. Hans G. Schlegel on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Beuscher, H.U., Andreesen, J.R. Eubacterium angustum sp. nov., a Gram-positive anaerobic, non-sporeforming, obligate purine fermenting organism. Arch. Microbiol. 140, 2–8 (1984). https://doi.org/10.1007/BF00409763
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00409763