Abstract
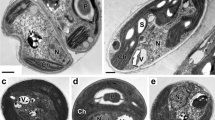
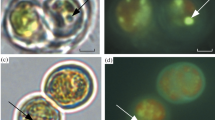
Acidic inorganic phosphate (Pi) pool (pH around 6) was detected besides the cytoplasmic pool in intact cells of Chlorella vulgaris 11h by 31P-in vivo nuclear magnetic resonance (NMR) spectroscopy. It was characterized as acidic compartments (vacuoles) in combination with the cytochemical technique; staining the cells with neutral red and chloroquine which are known as basic reagents specifically accumulated in acidic compartments. Under various conditions, the results obtained with the cytochemical methods were well correlated with those obtained from in vivo NMR spectra; the vacuoles were well developed in the cells at the stationary growth phase where the acidic Pi signal was detected. In contrast, cells at the logarithmic phase in which no acidic Pi signal was detected contained only smaller vesicles that accumulated these basic reagents. No acidic compartment was detected by both cytochemical technique and 31P-NMR spectroscopy when the cells were treated with NH4OH. The vacuolar pH was lowered by the anaerobic treatment of the cells in the presence of glucose, while it was not affected by the external pH during the preincubation ranging from 3 to 10. Possible vacuolar functions in unicellular algae especially with respect to intracellular pH regulation are discussed.
Similar content being viewed by others
Abbreviations
- EDTA:
-
ethylenediaminetetraacetic acid
- HEPES:
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- MDP:
-
methylene diphosphonic acid
- NMR:
-
nuelear magnetic resonance
- PCA:
-
perchloric acid
- PCV:
-
packed cell volume
- Pi:
-
inorganic phosphate
- Pic:
-
sytoplasmic inorganic phosphate
- Piv:
-
vacuolar inorganic phosphate
- ppm:
-
parts per million
- SP:
-
sugar phosphates
- TCA:
-
trichloroacetic acid
References
Atkinson Jr, AW, John PCL, Gunning BES (1974) The growth and division of single mitochondrion and other organelles during the cell cycle of Chlorella studied by quantitative stereology and three dimensional reconstitution. Protoplasma 81:77–109
Boller T, Wiemken A (1986) Dynamics of vacuolar compartmentation. Ann Rev Plant Physiol 37:137–164
Conn HJ (1969) H. J. Conn's biological stains, 8th edn. Williams and Wilkins, Baltimore, pp 269–271
Gehl K, Colman B (1985) Effect of external pH on the internal pH of Chlorella saccharophila. Plant Physiol 77:917–921
Huber-Wälchli V, Wiemken A (1979) Differential extraction of soluble pools from the cytosol and the vacuoles of yeast (Candida utilis) using DEAE-dextran. Arch Microbiol 120:141–149
Kowallik W, Gaffron H (1967) Enhancement of respiration and fermentation in algae by blue light. Nature 215:1038–1040
Labotka RJ, Kleps RA (1983) A phosphate-analogue probe of red cell pH using phosphorus-31 nuclear magnetic resonance. Biochemistry 22:6089–6095
Lane AE, Burris JE (1981) Effects of environmental pH on the internal pH of Chlorella pyrenoidosa, Scenedesmus quadricauda, and Euglena mutabilis. Plant Physiol 68:439–442
Lenz AG, Holzer H (1984) Effects of chloroquine on proteolytic processes and energy metabolism in yeast. Arch Microbiol 137:104–108
Matile Ph, Moor H, Robinow CF (1969) Yeast cytology. In: rose AH, Harrison JS (eds) The yeast, vol 1, Biology of yeasts. Academic Press, London, pp 219–302
Mimura T, Kirino Y (1984) Changes in cytoplasmic pH measured by 31P-NMR in cells of Nitellopsis obtusa. Plant Cell Physiol 25:813–820
Mitsumori F, Ito O (1984) Phosphorus-31 nuclear magnetic resonance studies of photosynthetic Chlorella. FEBS Lett. 174:248–252
Moriyasu Y, Shimmen T, Tazawa M (1984) Vacuolar pH regulation in Chara australis. Cell str Funct 9:225–234
Nicolay K, Scheffers WA, Bruineberg PM, Kaptein R (1982) Phosphorus-31 nuclear magnetic resonance studies of intracellular pH, phosphate compartmentation and phosphate transport in yeasts. Arch Microbiol 133:83–89
Ogasawara N, Miyachi S (1970) Effects of disalicylidenepropandiamine and near far-red light on 14C-fixation in Chlorella cells. Plant Cell Physiol 11:411–416
Oh-hama T, Siebelt F, Furihata K, Seto H, Miyachi S, Ohmori M (1986) 31P-NMR studies on inorganic polyphosphates in microalgae. J Phycol 22:485–490
Ohkuma S, Poole B (1981) Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol 90: 656–664
Ohmori M, Oh-hama T, Furihata K, Miyachi S (1986) Effect of ammonia on cellular pH of Anabaena cylindrica determined with 31P NMR spectroscopy. Plant Cell Physiol 27:563–566
Palevitz BA, O'kane DJ (1981) Epifluorescene and video analysis of vacuole motility and development in stomatal cells of Allium. Science 214:443–444
Poole B, Ohkuma S (1981) Effects of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 90:665–669
Roberts JKM (1984) Study of plant metabolism in vivo using NMR spectroscopy. Annu Rev Plant Physiol 35:375–385
Roberts JKM, Ray P, Wade-Jardetzky, N, Jardetzky O (1980) Estimation of cytoplasmic and vacuolar pH in higher plant cells by 31P NMR. Nature 283:870–872
Sianoudis J, Mayer A, Leibfritz D (1984) Investigation of intracellular phosphate pools of the green alga Chlorella using 31P nuclear magnetic resonance. Organic Magnetic Resonance 22:364–368
Sianoudis J, Küsel AC, Naujokat T, Offermann W, Mayer A, Grimme LH, Leibfritz D (1985) Respirational activity of Chlorella fusca monitored by in vivo P-31 NMR. Eur Biophys J 13:89–97
Syrett PJ, Wong H-A (1963) The fermentation of glucose by Chlorella vulgaris. Biochem J 89:308–315
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuchitsu, K., Oh-hama, T., Tsuzuki, M. et al. Detection and characterization of acidic compartments (vacuoles) in Chlorella vulgaris 11h cells by 31P-in vivo NMR spectroscopy and cytochemical techniques. Arch. Microbiol. 148, 83–87 (1987). https://doi.org/10.1007/BF00425353
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00425353