Abstract
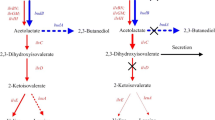
Growth of Klebsiella pneumoniae M5a1 on 3-hydroxybenzoate leads to the induction of 3-hydroxybenzoate monooxygenase, 2,5-dihydroxybenzoate dioxygenase, maleylpyruvate isomerase and fumarylpyruvate hydrolase. Growth in the presence of 2,5-dihydroxybenzoate also induces all of these enzymes including the 3-hydroxybenzoate monooxygenase which is not required for 2,5-dihydroxybenzoate catabolism. Mutants defective in 3-hydroxybenzoate monooxygenase fail to grow on 3-hydroxybenzoate but grow normally on 2,5-dihydroxybenzoate. Mutants lacking maleylpyruvate isomerase fail to grow on 3-hydroxybenzoate and 2,5-dihydroxybenzoate. Both kinds of mutants grow normally on 3,4-dihydroxybenzoate. Mutants defective in maleylpyruvate isomerase accumulate maleylpyruvate when exposed to 3-hydroxybenzoate and growth is inhibited. Secondary mutants that have additionally lost 3-hydroxybenzoate monooxygenase are no longer inhibited by the presence of 3-hydroxybenzoate. The 3-hydroxybenzoate monooxygenase gene (mhbM) and the maleylpyruvate isomerase gene (mhbI) are 100% co-transducible by P1 phage.
Similar content being viewed by others
References
Bayly RC, Chapman PJ, Dagley S, DiBerardino D (1980) Purification and some properties of maleylpyruvate hydrolase and fumarylpyruvate hydrolase from Pseudomonas alcaligenes. J Bacteriol 143: 70–77
Burlinghame R, Chapman PJ (1983) Catabolism of phenylpropionic acid and its 3-hydroxy derivative by Escherichia coli. J Bacteriol 155: 113–121
Cooper RA, Skinner MA (1980) Catabolism of 3- and 4-hydroxyphenylacetate by the 3,4-dihydroxyphenylacetate pathway in Escherichia coli. J Bacteriol 143: 302–306
Cooper RA, Jones DCN, Parrott S (1985) Isolation and mapping of Escherichia coli K12 mutants defective in phenylacetate degradation. J Gen Microbiol 131: 2753–2757
Czok R, Lamprecht W (1974) Pyruvate, phosphoenolpyruvate and d-glycerate 2-phosphate. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3, 2nd english edn. Verlag Chemie, Weinheim; Academic Press, New York London, pp 1446–1451
Dagley S (1975) A biochemical approach to some problems of environmental pollution. In: Campbell PN, Aldridge WN (eds) Essays in biochemistry, vol 11. Academic Press, London New York San Francisco, pp 81–138
Dagley S, Chapman PJ, Gibson DT (1965) The metabolism of β-phenylpropionic acid by an Achromobacter. Biochem J 97: 643–650
Deschamps AM, Richard C, Lebeault J-M (1983) Bacteriology and nutrition of environmental strains of Klebsiella pneumoniae involved in wood and bark decay. Ann Microbiol (Paris) 134A: 189–196
Dixon RA (1984) The genetic complexity of nitrogen fixation. J Gen Microbiol 130: 2745–2755
Doten RC, Ornston LN (1987) Protocatechuate is not metabolized via catechol in Enterobacter aerogenes. J Bacteriol 169: 5827–5830
Gornall AC, Bardawill CS, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177: 751–766
Grant DJW, Patel JC (1969) The non-oxidative decarboxylation of p-hydroxybenzoic acid, gentisic acid, protocatechuic acid and gallic acid by Klebsiella aerogenes (Aerobacter aerogenes). Antonie van Leuwenhoek 35: 325–343
Groseclose EE, Ribbons DW (1973) 3-Hydroxybenzoate 6-hydroxylase from Pseudomonas aeruginosa. Biochem Biophys Res Commun 55: 897–903
Gutmann I, Wahlefeld AW (1976) l−(-)-Malate. Determination with malate dehydrogenase and NAD. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 3, 2nd english edn. Verlag Chemie, Weinheim; Academic Press, New York London, pp 1585–1589
Hareland WA, Crawford RL, Chapman PJ, Dagley S (1975) Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol 121: 272–285
Jenkins JR, Cooper RA (1988) Molecular cloning, expression, and analysis of the genes of the homoprotocatechuate catabolic pathway of Escherichia coli C. J Bacteriol 170: 5317–5324
Lack L (1959) The enzymatic oxidation of gentisic acid. Biochim Biophys Acta 34: 117–123
Lack L (1961) Enzymatic cis-trans isomerization of maleylpyruvate. J Biol Chem 236: 2835–2840
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA
Nordmann J, Nordmann R (1969) Organic acids. In: Smith I (ed) Chromatographic and electrophoretic techniques, 3rd edn. William Heinemann, London, pp 342–363
Parrott S, Jones S, Cooper RA (1987) 2-Phenylethylamine catabolism by Escherichia coli K12. J Gen Microbiol 133: 347–351
Patel JC, Grant DJW (1969) The formation of phenol in the degradation of p-hydroxybenzoic acid by Klebsiella aerogenes (Aerobacter aerogenes). Antonie van Leuwenhoek 35: 53–64
Skinner MA, Cooper RA (1982) An Escherichia coli mutant defective in the NAD-dependent succinate semialdehyde dehydrogenase. Arch Microbiol 132: 270–275
Smith I, Seakins JWT, Dayman J (1969) Phenolic acids. In: Smith I (ed) Chromatographic and electrophoretic techniques, 3rd edn. William Heinemann, London, pp 364–389
Sparnins VL, Chapman PJ, Dagley S (1974) Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J Bacteriol 120: 159–167
Streicher S, Gurney E, Valentine RC (1971) Transduction of the nitrogen-fixation genes in Klebsiella pneumoniae. Proc Natl Acad Sci USA 68: 1174–1177
Wheelis ML, Palleroni NJ, Stanier RY (1967) The metabolism of aromatic compounds by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol 59: 302–314
Yano K, Arima K (1958) Metabolism of aromatic compounds by bacteria, II. m-Hydroxybenzoic acid hydroxylase A and B; 5-dehydroshikimic acid, a precursor of protocatechuic acid, a new pathway from salicylic acid to gentisic acid. J Gen Appl Microbiol 4: 241–258
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jones, D.C.N., Cooper, R.A. Catabolism of 3-hydroxybenzoate by the gentisate pathway in Klebsiella pneumoniae M5a1. Arch. Microbiol. 154, 489–495 (1990). https://doi.org/10.1007/BF00245233
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00245233