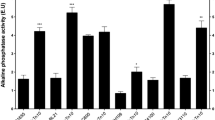
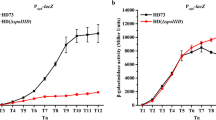
Abstract
The presence of the mutant prophage ϕ105cts23 in Bacillus subtilis strains strongly affected several biological parameters including the viability of protoplasts and the establishment of plasmid pC194. A defective inducibility of the prophage after treatments that de-repress the SOS-like response were also observed. Although these alterations suggested a Rec-deficient phenotype, homologous recombination was not impaired in these lysogenic derivatives. In fact, chromosomal DNA transformation in these competent cells was more efficient than in cells carrying the wild type prophage: cell death due to prophage induction upon competence development was lower than expected. Alterations in the response to SOS-inducing agents and to osmotic stress correlated with the presence of this particular mutant prophage or the cloned thermosensitive repressor at the permissive temperature. The induction of an anti-SOS effect is discussed.
Similar content being viewed by others
References
Alonso J, Viret J, Tailor R (1987) Plasmid maintenance in Bacillus subtilis recombination-deficient mutants. Mol Gen Genet 208: 349–352.
Alonso J, Tailor R, Lüder G (1988) Characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol 170: 3001–3007.
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81: 741–746.
Armentrout R, Rutberg L (1970) Mapping of prophage and mature deoxyribonucleic acid from temperate Bacillus bacteriophage ϕ105 by marker rescue. J Virol 6: 760–767.
Bagdasarian M, Bailone A, Bagdasarian MM, Manning P, Lurz R, Timmis K, Devoret R (1986) An inhibitor of SOS induction specified by a plasmid locus in Escherichia coli. Proc Natl Acad Sci USA 83: 5723–5726.
Bagdasarian M, Bailone A, Angulo J, Scholz P, Bagdasarian M, Devoret R (1992) PsiB, an Anti-SOS protein is transiently expressed by the F sex factor during its transmission to an Escherichia coli K-12 recipient. Mol Microbiol 6: 885–895.
Bailone A, Bäckman A, Sommer S, Celerier J, Bagdasarian MM, Bagdasarian M, Devoret R (1988) PsiB polypeptide prevents activation of RecA protein in Escherichia coli. Mol Gen Genet 214: 389–395.
Birnboim H, Doly J (1979) A rapid alkaline procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7: 1513–1517.
Cully D, Garro A (1980) Expression of superinfection immunity to bacteriophage ϕ105 by Bacillus subtilis cells carrying a plasmid chimera of pUB110 and EcoRI fragment F of ϕ105 DNA. J Virol 34: 789–791.
Cully D, Garro A (1985) Nucleotide sequence of the immunity region of Bacillus subtilis bacteriophage ϕ105: identification of the repressor gene and its mRNA and protein product. Gene 38: 153–164.
Dhaese P, Seurinck J, Desmet B, VanMontagu M (1985) Nucleotide sequence and mutational analysis of an immunity repressor gene from Bacillus subtilis temperate phage ϕ105. Nucleic Acids Res 13: 5441–5455.
Errington J (1984) Efficient Bacillus subtilis cloning system using bacteriophage vector ϕ105 J9. J Gen Microbiol 130: 2615–2628.
Friedman B, Yasbin A (1983) The genetics and specificity of the constitutive excision repair system of Bacillus subtilis. Mol Gen Genet 190: 481–486.
Golub E, Bailone A, Devoret R (1988) A gene encoding an SOS inhibitor is present in different conjugative plasmids. J Bacteriol 170: 4392–4394.
Gros M, Riele Hte, Ehrlich SD (1987) Rolling circle replication of single stranded DNA plasmid pC194. EMBO J 6: 3863–3869.
Gruss A, Ehrlich SD (1989) The family of highly interrelated single stranded DNA plasmids. Microbiol Rev 53: 231–241.
Hartford N, Samojlenko J, Mergery M (1973) Isolation and characterization of recombination defective mutants of Bacillus subtilis. In: Archer L (ed) Bacterial transformation. Academic Press, London, pp 241–267.
Iordanescu A (1975) Recombinant plasmid obtained from two different compatible staphylococcal plasmids. J Bacteriol 124: 97–601.
Jones AL, Barth P, Wilkins B (1992) Zygotic induction of plasmid ssb and psiB genes following conjugative transfer of Incl1 plasmid Collb-P9. Mol Microbiol 6: 605–613.
Lepesant-Kejzlorova J, Lepesant J, Walle J, Billaultand A, Dedonder R (1975) Revision of the linkage map of Bacillus subtilis 168: indications for circularity of the chromosome. J Bacteriol 121: 823–834.
Lijima T, Kawanura F, Saito H, Ikeda Y (1980) A specialized transducing phage constructed from Bacillus subtilis phage ϕ105. Gene 9: 115–126.
Little J, Mount D (1982) The SOS regulatory system of Escherichia coli. Cell 29: 11–22.
Love P, Yasbin R (1984) Characterization of the indicible SOS-like system of Bacillus subtilis. J Bacteriol 160: 910–920.
Love P, Yasbin R (1986) Induction of the B. subtilis SOS-like response by E. coli RecA protein. Proc Natl Acad Sci USA 83: 5204–5208.
Lovett CM, Love PE, Yasbin RE (1989) Competence specific induction of the Bacillus subtilis RecA protein analog: evidence for dual regulation of a recombination protein. J Bacteriol 171: 2318–2322.
Marrero R, Yasbin R (1988) Cloning of the Bacillus subtilis recE+ gene and functional expression of recE+ in Bacillus subtilis. J Bacteriol 170: 335–344.
Marsh L, Walker GC (1987) New phenotypes associated with mucAB: alteration of a mucA sequence homologous to the LexA cleavage site. J Bacteriol 169: 1818–1823.
Mazza G, Galizzi A (1978) The genetics of DNA replication repair and recombination in Bacillus subtilis. Microbiologia 1: 111–135.
Mazza G, Fortunato A, Ferrari E, Canosi U, Falaschi A, Polsinelli M (1975) Genetic and enzymatic studies in the recombination process in Bacillus subtilis. Mol Gen Genet 136: 9–30.
Niaudet B, Ehrlich SD (1979) In vitro genetic labeling Bacillus subtilis cryptic plasmid pHV400. Plasmid 2: 48–58.
Osburne M, Craig R, Rothstein D (1985) Thermoinducible transcription system for Bacillus subtilis that utilizes control elements from temperate phage ϕ105. J Bacteriol 166: 1101–1108.
Raymond-Denise A, Guillen N (1992) Expression of the Bacillus subtilis dinR and RecA genes after DNA damage and during competence. J Bacteriol 174: 3171–3176.
Rubinstein C, Moratinos L, Coso O, Sanchez-Rivas C (1988) Improvements in the transformation of Bacillus subtilis protoplasts by plasmid DNA. FEMS Microbiol Lett 56: 67–70.
Rutberg L (1969) Mapping of a temperate bacteriophage active in Bacillus subtilis. J Virol 3: 38–44.
Sanchez-Rivas C, Garro A (1979) Bacterial fusion assayed by a prophage complementation test. J Bacteriol 137: 1340–1345.
Schaeffer P, Millet J, Aubert J (1965) Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA 54: 701–711.
Schaeffer P, Cami B, Hotchkiss R (1975) Fusion of bacterial protoplasts. Proc Natl Acad Sci USA 77: 2151–2155.
Scher B, Lalu M, Garro A (1978) Correlates genetic and EcoRI cleavage map of Bacillus subtilis bacteriophage 105 DNA. J Virol 28: 395–402.
Vosde W, Venema G (1983) Transformation of Bacillus subtilis competent cells: identification and regulation of the recE gene product. Mol Gen Genet 190: 56–64.
Vosde W, Vries S, Venema G (1983) Cloning and expression of the Escherichia coli recA gene in Bacillus subtilis. Gene 25: 301–308.
Wojciechowski MF, Peterson KR, Love PE (1991) Regulation of the SOS response in Bacillus subtilis: evidence for a LexA repressor homolog. J Bacteriol 173: 6489–6498.
Yasbin R (1977a) DNA repair in Bacillus subtilis. I. The presence of an inducible system. Mol Gen Genet 153: 211–218.
Yasbin R (1977b) DNA repair in Bacillus subtilis. II. Activation of the inducible system in competent bacteria. Mol Gen Genet 153: 219–225.
Yasbin R, Anderson B (1982) DNA repair in Bacillus subtilis. The identification of a strain temperature inducible for SOS function. In: Ganesan AR, Hoch JA, Chang S (eds) Molecular cloning and gene regulation in the bacilli. Academic Press, New York, pp 237–247.
Yasbin R, Fields P, Anderson B (1980) Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene 12: 155–159.
Yasbin R, Cheo D, Bayles K (1992) Inducible DNA repair and differentiation in Bacillus subtilis: interactions between global regulons. Mol Microbiol 6: 1263–1270.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rubinstein, C.P., Coso, O.A., Ruzal, S. et al. Anti-SOS effects induced in Bacillus subtilis by a ϕ105 mutant prophage. Arch. Microbiol. 160, 486–491 (1993). https://doi.org/10.1007/BF00245310
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00245310