Abstract
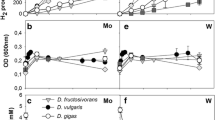
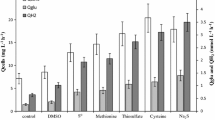
Growth of Desulfovibrio gigas NCIMB 9332 in mineral, vitamin-supplemented media with ethanol as substrate was strongly stimulated by the addition of tungstate (optimal level approximately 10-7 M). At suboptimal tungstate concentrations, up to 1.0 mM acetaldehyde was detected in the culture supernatant and growth was slow. Omission of both tungstate and molybdate from the media prevented growth and ethanol utilization. Tungstate-deprived cultures that were grown on lactate had much lower aldehyde dehydrogenase (benzylviologen as acceptor; BV-AIDH) levels than tungstate-supplemented cultures. These data suggest that tungstate is required for the synthesis of active BV-AIDH. The characteristics of the enzyme activities in cell-free extracts show that the BV-AIDH activity present in tungstate-supplemented cultures is not due to the recently characterized molybdenum-containing aldehyde dehydrogenase of D. gigas. Out of 13 other strains of ethanol-oxidizing, gram-negative, sulfate-reducing bacteria tested, most strains grew well with either tungstate or molybdate supplementation. In contrast to a recent report, good growth on ethanol of two D. baculatus (Desulfomicrobium) strains (DSM 1741 and DSM 1743) was observed.
Similar content being viewed by others
Abbreviations
- BV-AIDH :
-
Benzylviologen-linked aldehyde dehydrogenase
- DCPIP-AIDH :
-
2,6-dichlorophenolindophenol-linked aldehyde dehydrogenase
- DTT :
-
dithiothreitol
References
Andreesen JR, Ljungdahl L (1973) Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol 116: 867–873
Barata BAS, LeGall J, Moura JJG (1993) Aldehyde oxidoreductase activity in Desulfovibrio gigas: in vitro reconstitution of an electron-transfer chain from aldehydes to the production of molecular hydrogen. Biochemistry 32: 11559–11568
Bradford MM, (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Devereux R, He S-H, Doyle CL, Orkland S, Stahl DA, LeGall J, Whitman WB (1990) Diversity and origin of Desulfovibrio species: phylogenetic definition of a family. J Bacteriol 172: 3609–3619
Esnault G, Caumette P, Garcia J-L (1988) Characterization of Desulfovibrio giganteus sp. nov., a sulfate-reducing bacterium isolated from a brackish coastal lagoon. Syst Appl Microbiol 10: 147–151
Gírio FM, Marcos JC, Amaral-Collaço MT (1992) Transition metal requirement to express high level NAD+-dependent formate dehydrogenase from a serine-type methylotrophic bacterium. FEMS Microbiol Lett 97: 161–166
Heijthuijsen JHFG, Hansen TA (1989) Anaerobic degradation of betaine by marine Desulfobacterium strains. Arch Microbiol 152: 393–396
Hensgens CMH, Vonck J, Van Beeumen J, Bruggen EFJ van, Hansen TA (1993) Purification and characterization of an oxygen-labile, NAD-dependent alcohol dehydrogenase from Desulfovibrio gigas. J Bacteriol 175: 2859–2863
Johnson JL, Rajagopalan KV, Mukund S, Adams MWW (1993) Identification of molybdopterin as the organic component of the tungsten cofactor in four enzymes from hyperthermophilic archaea. J Biol Chem 268: 4848–4852
Kremer DR, Hansen TA (1987) Glycerol and dihydroxyacetone dissimilation in Desulfovibrio strains. Arch Microbiol 147: 249–256
Kremer DR, Nienhuis-Kuiper HE, Hansen TA (1988) Ethanol dissimilation in Desulfovibrio. Arch Microbiol 150: 552–557
Laanbroek HJ, Abee T, Voogd IL (1982) Alcohol conversions by Desulfobulbus propionicus Lindhorst in the presence and absence of sulfate and hydrogen. Arch Microbiol 133: 178–184
Mukund S, Adams MWW (1991) A novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. J Biol Chem 266: 14208–14216
Mukund S, Adams MWW (1993) Characterization of a novel tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon, Thermococcus litoralis. J Biol Chem 268: 13592–13600
Oppenberg B, Schink B (1990) Anaerobic degradation of 1,3-propancdiol by sulfate-reducing and fermenting bacteria. Antonie van Leeuwenhoek 57: 205–213
Pfennig N, Lippert KD (1966) Über das Vitamin B12-Bedürfnis phototropher Schwefelbakterien. Arch Mikrobiol 55: 245–256
Schmitz RA, Albracht SPJ, and Thauer RK (1992a) Properties of the tungsten-substituted molybdenum formylmethanofuran dehydrogenase from Methanobacterium wolfei. FEBS Lett 1: 78–81
Schmitz RA, Albracht SPJ, Thauer RK (1992b) A molybdenum and a tungsten isoenzyme of formylmethanofuran dehydrogenase in the thermophilic archaeon Methanobacterium wolfei. Eur J Biochem 209: 1013–1018
Stams AJM, Veenhuis M, Weenk GH, Hansen TA (1983) Occurrence of polyglucose as a storage polymer in Desulfovibrio species and Desulfobulbus propionicus. Arch Microbiol 136: 54–59
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA (1984) Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139: 167–173
Strobl G, Feicht R, White H, Lottspeich F, Simon H (1992) The tungsten-containing aldehyde oxidoreductase from Clostridium thermoacetium and its complex with a viologen-accepting NADPH oxidoreductase. Biol Chem Hoppe Seyler 373: 123–132
Szewzyk R, Pfennig N (1990) Competition for ethanol between sulfate-reducing and fermenting bacteria. Arch Microbiol 153: 470–477
Turner N, Barata B, Bray RC, Deistung J, Le Gall J, Moura JJG (1987) The molybdenum iron-sulfur protein from Desulfovibrio gigas as a form of aldehyde oxidase. Biochem J 243: 755–761
White H, Simon H (1992) The role of tungstate and/or molybdate in the formation of aldehyde oxidoreductase in Clostridium thermoaceticum and other acetogens; immunological distances of such enzymes. Arch Microbiol 158: 81–84
White H, Strobl G, Simon H (1989) Carboxylic acid reductase: a new tungsten enzyme catalyses the reduction of non-activated carboxylic acids to aldehydes. Eur J Biochem 184: 89–96
Widdel F (1986) Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol 51: 1056–1062
Widdel F (1988) Microbiology and ecology of sulfate-and sulfur-reducing bacteria. In: Zehnder AJB (ed) Biology of anaerobic organisms. Wiley, New York, pp 469–585
Widdel F, Bak F (1991) Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (eds) The prokaryotes, 2nd edn. Springer, Berlin Heidelberg New York, pp 3352–3378
Winter J, Lerp C, Zabel H-P, Wildenauer FX, König H, Schindler F (1984) Methanobacterium wolfei, sp. nov., a new tungsten-requiring, thermophilic, autotrophic methanogen. Syst Appl Microbiol 5: 457–461
Yamamoto I, Saiki T, Liu S-M, Ljungdahl LG (1983) Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem 258: 1826–1832
Zellner G, Jargon A (1993) Characterization of aldehyde dehydrogenase in crude extracts of Desulfovibrio simplex and the role of tungstate. Bioengineering 9: 46 (P129)
Zellner G, Alten C, Stackebrandt E, Conway de Macario E, Winter J (1987) Isolation and characterization of Methanocorpusculum parvum gen. nov., spec. nov., a new tungsten requiring, coccoid methanogen. Arch Microbiol 147: 13–20
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hensgens, C.M.H., Nienhuis-Kuiper, M.E. & Hansen, T.A. Effects of tungstate on the growth of Desulfovibrio gigas NCIMB 9332 and other sulfate-reducing bacteria with ethanol as a substrate. Arch. Microbiol. 162, 143–147 (1994). https://doi.org/10.1007/BF00264388
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00264388