Abstract
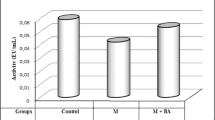
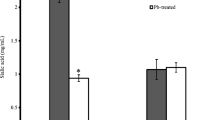
The well known fact that the activity of δ-aminolevulinic acid dehydratase (ALAD: EC 4.2.1.24) is reduced in red cells of animals with lead poisoning was found to be upset, by using a modified method of Gibson's original procedure, for determination of activated ALAD activity.
The modified method involves addition of 0.2 mM Zn2+ and then preheating the enzyme solution at 60° C for 5 min before following Gibson's original procedure. With this methodological modification, the ALAD activity of erythrocytes of rats poisoned with lead was found increased. Furthermore, the enzyme was purified from the peripheral blood of lead-poisoned rats. ALAD protein in peripheral blood was also determined by single radial immuno diffusion using rabbit anti-serum raised against rat liver ALAD. As the result, the ALAD activity obtained from the modified method was found to be directly proportional to the absolute amount of enzyme proteins determined both by chemically and immunochemically.
The modified method for measuring true ALAD content in blood cells in lead poisoning is more reliable than previous ones.
Similar content being viewed by others
References
Abdulla M, Svensson S, Haeger-Aronsen B (1979) Antagonistic effects of zinc and aluminum on lead inhibition of δ-aminolevulinic acid dehydratase. Arch Environ Health 34: 464–469
Anderson PM, Desnick RJ (1979) Purification and properties of δ-aminolevulinate dehydrase from human erythrocytes. J Biol Chem 254:6924–6930
Bevan DR, Bodlaender P, Shemin D (1980) Mechanism of porphobilinogen synthase. J Biol Chem 255:2030–2035
Burch HB, Siegel AL (1971) Improved method for measurement of deltaaminolevulinic acid dehydratase activity of human erythrocytes. Clin Chem 17:1038–1041
Cheh A, Neilands JB (1973) Zinc, an essential metal ion for beef liver δ-aminolevulinate dehydratase. Biochem Biophys Res Commun 55:1060–1063
Chiba M (1976) Activity of erythrocyte δ-aminolevulinic acid dehydrase and its change by heat treatment as indices of lead expodure. Br J Ind Med 33:36–42
Davis JR, Avram MJ (1978) Developmental changes in delta-aminolevulinic acid dehydratase (ALAD) activity and blood reticulocyte percent in the developing rat. Mech Ageing Dev 7:123–129
Doyle D, Schimke RT (1969) The genetic and developmental regulation of hepatic δ-aminolevulinate dehydratase in mice. J Biol Chem 244: 5449–5459
Dresel EIB, Falk JE (1956) Studies on the biosynthesis of blood pigment, 2) haem and porphyrin formation in intact chiken erythrocytes. Biochem J 63:72–79
Finelli VN, Klauder DS, Karaffa MA, Petering HG (1975) Interaction of zinc and lead on δ-aminolevulinate dehydratase. Biochem Biophys Res Commun 65:303–311
Gelman BB, Michaelson IA, Bus JS (1978) The effect of lead on oxidative hemolysis and erythrocyte defense mechanisms in the rat. Toxicol Appl Pharmacol 45:119–129
Gibson KD, Neuberger A, Scott JJ (1955) The purification and properties of δ-aminolaevulic acid dehydrase. Biochem J 61: 618–629
Granick S, Mauzerall D (1958) Porphyrin biosynthesis in erythrocytes II. J Biol Chem 232:1119–1139
Granick JL, Sassa S, Granick S, Levere RD, Kappas A (1973) Studies in lead poisoning II. Correlation between the ratio of activated to inactivated δ-aminolevulinic acid dehydratase of whole blood and the blood lead level. Biochem Med 8:149–159
Gurne D, Chen J, Shemin D (1977) Dissociation and reassociation of immobilized porphobilinogen synthase: Use of immobilized subunits for enzyme isolation. Proc Natl Acad Sci USA 74:1383–1387
Haeger-Aronsen B, Abdulla M, Fristedt BI (1971) Effect of lead on δ-aminolevulinic acid dehydrase activity in red blood cells. Arch Environ Health 23:440–445
Hernberg S, Nikkanen J, Melling G, Lilius H (1970) δ-aminolevulinic acid dehydrase as a measure of lead exposure. Arch Environ Health 21:140–145
Hirano H, Omichi M, Ishikawa K (1979) ALAD activity of red cells of different ages. Jpn J Ind Health 21: 55–60
Kajimoto M, Hasegawa Y, Suzuki T, Kondo M, Kimura H, Niwa M, Urata G (1981) Effect of iron and protein deficiency on hemoglobin content, hematocrit, reticulocytes and tissue iron levels of growing rats. J Jpn Soc Food Nutr 34:43–49
Kondo M, Kajimoto M, Sasaki A, Niwa M, Suzuki T, Kimura H, Urata G (1980) Elevation of ALAD in lead poisoning. In: Suzuki T (ed) Biochemical approach to toxicology. Shinohara shuppan. Tokyo, pp 179–197
Kondo M, Kajimoto M, Kimura H, Suzuki T, Sasaki A, Niwa M, Urata G (1981) A specific inhibitor to δ-aminolevulinate dehydratase in rat bone marrow cells. Arch Biochem Biophys 208:189–194
Labbe RF, Finch CA (1977) Effects of iron status on δ-aminolevulinic acid dehydratase activity. Biochem Med 18:323–329
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Maes J, Gerber GB (1978) Increased ALA dehydratase activity and spleen weight in lead-intoxicated rats. A consequence of increased blood cell destruction. Experientia 34:381–382
Mancini G, Cardonara AO, Heremans JF (1965) Immunochemical quantitation of antigens by single radual immuno diffusion. Immunochemical 2:235–254
National Academy of Sciences (1972) Biologic effects of atmospheric pollutants. Lead; airborne lead in perspective. National Academy of Sciences, Washington, DC, pp 1–313
Niwa M, Kajimoto M, Sasaki A, Kondo M, Urata G (1978) Influence of protein diet and age on ALA-D in lead poisoning. Jpn J Hyg 33:153
Ornstein L (1964) Disc electrophoresis I. Background and theory. Ann NY Acad Sci 121:321–349
Sakai T, Yanagihara S, Ushio K (1980) Restoration of lead-inhibited 5-aminolevulinate dehydratase activity in whole blood by heat, zinc ion, and(or) dithiothreitol. Clin Chem 26: 625–628
Shemin D (1976) 5-Aminolaevulinic acid dehydratase: structure, function, and mechanism. Phillos Trans R Soc Lond B 273:109–115
Schlick K, Friedberg (1980) The action of small doses of lead on erythrocyte δ-aminolevulinic acid dehydratase in the mouse. Arch Toxicol 43:213–220
Thomasino JA, Zuroweste E, Brooks SM, Petering HG, Lerner SI, Finelli VN (1977) Lead, zinc, and erythrocyte δ-aminolevulinic acid dehydratase: relationships in lead toxicity. Arch Environ Health 32:244–247
Tomokuni K (1974) δ-Aminolevulinic acid dehydratase test for lead exposure. Arch Environ Health 29:274–281
Tsukamoto I, Yoshinaga T, Sano S (1979) The role zinc with special reference to the essential thiol groups in δ-aminolevulinic acid dehydratase of bovine liver. Biochim Biophys Acta 570:167–178
Urata G, Kondo M, Kimura H, Hasegawa Y, Suzuki T, Kajimoto M (1981) Effect of iron and protein deficiency on ALA dehydratase ALA-synthetase and Heme-synthetase activities of growing rats. J Jpn Soc Food Nutr 34: 51–58
Wada O, Takeo K, Yano Y, Ono T, Nagahashi M, Seki H (1976) δ-Aminolevulinic acid dehydratase in low level lead exposure. Arch Environ Health 31: 211–215
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kajimoto, M., Kondo, M., Niwa, M. et al. Increase of δ-Aminolevulinic Acid Dehydratase (ALAD) in rat erythrocytes in lead poisoning. Arch Toxicol 52, 1–11 (1983). https://doi.org/10.1007/BF00317977
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00317977