Abstract
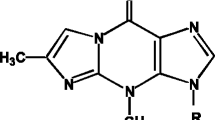
N-Nitroso-N-(3-keto-1,2-butanediol)-3′-nitrotyramine (NO-NTA) is a product of a model browning system in the presence of sodium nitrite. In this study, the chemical structure is confirmed by spectral studies, including UV, mass spectrometry, nuclear magnetic resonance and infrared spectroscopy. NO-NTA is strongly genotoxic to the rat hepatocyte and is moderately cytotoxic to mouse C3H10T1/2 cells. Results obtained in this study indicate that NO-NTA inflicted DNA damage through the formation of a DNA adduct. In addition, C3H10T1/2 cells were treated with NO-NTA and, following addition of 12-O-tetradecanoylphorbol-13-acetate (TPA) as promotor, the increase of transformed foci indicated that NO-NTA could possibly be an inhibitor of TPA tumor promotion. A transformed cell line from NO-NTA initiated and TPA promoted foci increased saturation density and growth ability in soft agar reactive to the control line. These results suggest that the formation of a genotoxic agent of nitroso-derivatives may take place in a nitrite-containing food system during processing and cooking.
Similar content being viewed by others
References
Bonney VR, Becker JE, Walker PR, Potter VR (1974) Primary monolayer cultures of adult rat liver parenchymal cells suitable for study of the regulation of enzyme synthesis. In Vitro 9: 399–413
Calaf G, Russo J (1993) Transformation of human breast epithelial cells by chemical carcinogens. Carcinogenesis 14: 483–493
Cheng SJ, Sala M, Li MH, Courtois J, Chouroulinkov J (1981) Promoting effect of Roussin’s red identified in pickled vegetable from Linxian China. Carcinogenesis 2: 313–319
Dipaolo JA, Takano K, Popescu NC (1972) Quantitation of chemically induced neoplastic transformation fo BALB/3T3 cloned cell lines. Cancer Res 32: 2686–2695
Dworschak E (1980) Nonenzymatic browning and its effect on protein nutrition. CRC Crit Rev Food Sci Nutr 12: 1–40
Freedman V, Shin SI (1974) Cellular tumorgenicity in nude mice correlation with cell growth in semi-solid medium. Cell 3: 355–359
Heyns KH, Roper SR (1979) N-Nitroso sugar amino acids. Angewandte Chemie (International Edition in English) 18: 878–880
Hsia MTS, Kreamerand BL, Dolara P (1983) A rapid and simple method to Quantitate chemically induced unscheduled DNA synthesis in freshly isolated rat hepatocytes facilitated by DNA retention of membrane filters. Mutat Res 122: 177–185
Landolph JR (1985) Chemical transformation in C3H10T1/2 C18 mouse embryo fibroblasts: historical background, assessment of the transformation assay, and evolution and optimization of the transformation assay protocol. IARC Scientific Publication No. 67, Transformation assay of established cell lines. Mechanism and Application. IARC, Lyon, pp 185–188
Lillehang JR, Dijurhuus R (1982) Effect of diethyl-stibestrol on the tranformable mouse embryo fibroblast C3H10T1/2 Cl 8 cells. Tumor promotion, cell growth, DNA synthesis, and ornithine decarboxylase. Carcinogenesis 3: 797–799
Lin JK (1990) Nitrosamines as potential environmental carcinogens in man. Clin Biochem 23: 67–72
Lin JK, Huang TY (1990) Mutagenicity and cytotoxicity of nitro and nitroso-compounds derived from the reaction of acetaminophen with nitrite. J Cheknese Biochem Soci 19: 25–36
Lin JK, Kuo ML (1990) Induction of ouabain-resistance mutation and cycle dependent transformation of C3H10T1/2 cells by N-nitroso-2-acetylaminofluorene. Mutat Res 230: 35–41
Lin JK, Lai CC (1982) Enhancement of Lactobacillus antacids and antibiotics on the level of nitrite in the gastro-intestinal tract of rats fed sodium nitrate. Food Chem Toxicol 20: 197–204
Magee PN, Barnes JM (1976) Carcinogenic nitroso compounds. Adv Cancer Res 12: 163–246
Maillard LC (1912) Action des acids amines sur les sucres: formation des melanoidines par voie methodique. CR Hebd, Seances Acad Sci 154: 66–68
Male R, Lillehang HR, Djurhuus R, Pryme IF (1985) In vitro transformation and tumor promotion studies of styrene and styrene oxide. Carcinogenesis 6: 1367–1370
Male R, Bjerkvig R, Lillehaug JR (1987) Biological and biochemical characterization of cell lines derived from initiation-promotion transformed C3H10T1/2 cells. Carcinogenesis 8: 1375–1383
Mihara S, Shibamoto T (1980) Mutagenicity of products obtained from cysteamine-glucose browning model systems. J Agric Food Chem 28: 62–66
Mondal S, Brankow DW, Heideverger C (1976) Two stage chemical oncogenesis in cultures on C3H10T/12, cells, Cancer Res 36: 2254–2260
Montesano R, Bartsch H (1976) Mutagenic and carcinogenic N-nitroso compounds: possible environmental hazards. Mutat Res 32: 179–228
Mosmann T (1983) Rapid colorimetric assay for growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 65: 55–63
Omura HN, Shinohara K, Murakami H (1983) Formation of mutagens by the Maillard reaction, In: Waller GR, Feather MS (eds) The Maillard reaction in foods and nutrition. American Chemical Society, Washington, DC, ACS Series 215, pp 537–563
Pintauro SJ, Page GV, Solberg, M, Lee TC, Chichester CO (1980) Absence of mutagenic response from extracts of Maillard browned egg albumin. J Food Sci. 45: 1442–1443
Pool BL, Roper H, Roper S, Romruen K (1984) Mutagenicity studies on N-nitrosated products of the Maillard browning reaction: N-nitroso-fructose-amino acids. Food Chem Toxicol 22: 797–801
Powrie W, Wu CH, Rosin M, Stich HF (1981) Clastogenic and mutagenic activities of Maillard reaction model systems. J Food Sci 46: 1433–1438
Reznikoff CA, Bertram JS, Brankow DW, Heiderberger C (1973) Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res 33: 3239–3249
Roper H, Roper S, Heyns K, Meyer B (1982) N-nitroso sugar amino acids (N-NO-fructose-l-amino acids) In: Bartsch H O’Neill IK, Castegnaro M, Okad M (eds) N-Nitroso compounds. Occurrence and biological effects. International Agency for Research on Cancer, Lyon, no. 41, p 87
Russell GF (1983) Nitrite interactions in model Maillard browning systems In: Walter GR, Feather MS (eds) The Maillard reaction in foods and nutrition. American Chemical Society, Washington, DC, ACS Series, 215, pp 83–90
Shank, RC (1975) Toxicology of N-nitroso compounds. Toxicol Appl Pharmacol 31: 361–368
Shibamoto T (1989) Genotoxicity testing of Maillard reaction products. Prog Clin Biol Res 304: 359–376
Spingarn NW, Garvie CT (1979) Formation of mutagens in sugarammonia model system. J Agric Food Chem 27, 1319–1321
Tannenraum SR, Weisman M, Fett D (1976) The effect of nitrate intake on nitrite formation. Food Cosmet Toxicol 14: 549–552
Vytasek R (1982) A sensitive fluorometric assay for the determination of DNA. Anal Biochem 120: 243–248
Wang CJ, Lin YL, Lin JK (1994) Mutagenicity and cytotoxicity of nitropyrrole compounds derived from the reaction of 2-acetyl pyrrole with nitrite. Food Chem Toxicol 32[9]: 839–844
Medzicha BL, Hill D, Cockshott TB (1982) The reactivity of intermediates in non-eznymatic browning reaction towards nitrite ion. J Sci Food Agric 33: 306–308
Yen GC, Lee TC (1988) Mutagenicity of Maillard browning reaction products from various nitrosated amino acid-glucose mixtures. Proc Natl Sci. Counc B ROC 12: 14–20
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, C.J., Huang, H.P., Tseng, T.H. et al. N-Nitroso-N-(3-keto-1,2-butanediol)-3′-nitrotyramine. Arch Toxicol 70, 10–15 (1995). https://doi.org/10.1007/s002040050242
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002040050242