Summary
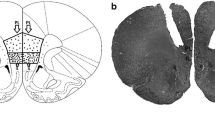
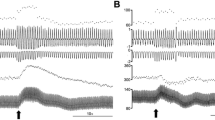
We localized glutamate-sensitive sites in the ventrolateral medulla of the rat with the spinal cord cut at C1. When unilaterally injected into a circumscribed region of the caudal ventrolateral medulla,l-glutamate (30–300 ng) elicited a dose-dependent increase in arterial pressure. The pressor response was accounted for by an increased release of vasopressin because it was abolished by the intravenous injection of a vasopressin antagonist. Bilateral microinjections of kainic acid (50 ng) into the ventrolateral glutamate-sensitive area markedly reduced a vasopressin-induced pressor response to kainic acid (30 ng), injected bilaterally into the nucleus tractus solitarii. It is concluded that the glutamate-sensitive neurons in the caudal ventrolateral medulla are involved in mediation of the vasopressin-induced pressor response arising from the nucleus tractus solitarii.
Similar content being viewed by others
References
Arnauld E, Czernichow P, Fumoux F, Vincent J-D (1977) The effects of hypotension and hypovolaemia on the liberation of vasopressin during haemorrhage in the unanaesthetized monkey. Pflügers Arch 371:193–200
Bisset GW, Feldberg W, Guertzenstein PG, Rocha E, Silva M Jr (1975) Vasopressin release by nicotine: the site of action. Br Pharmacol 54:463–474
Blessing WW, Reis DJ (1982) Inhibitory cardiovascular function of neurons in the caudal ventrolateral medulla of the rabbit: relationship to the area containing A1 noradrenergic cells. Brain Res 253:161–171
Blessing WW, Sved AF, Reis DJ (1982) Destruction of noradrenergic neurons in rabbit brainstem elevates plasma vasopressin, causing hypertension. Science 217:661–663
Blessing WW, Sved AF, Reis DJ (1984) Arterial pressure and plasma vasopressin: regulation by neurons in the caudal ventrolateral medulla of the rabbit. Clin Exp Hyper A 6:149–156
Ciriello J, Calaresu FR (1980a) Autoradiographic study of ascending projections from cardiovascular sites in the nucleus tractus solitarii in the cat. Brain Res 186:448–453
Ciriello J, Calaresu FR (1980b) Monosynaptic pathway from cardiovascular neurons in the nucleus tractus solitarii to the paraventricular nucleus in the cat. Brain Res 193:529–533
Ciriello J, Caverson MM (1984) Direct pathways from neurons in the ventrolateral medulla relaying cardiovascular afferent information to the supraoptic nucleus in the cat. Brain Res 292:221–228
Clark BJ, Rocha E, Silva M (1967) An afferent pathway for the selective release of vasopressin in response to carotid occulusion and haemorrhage in the cat. J Physiol 191:529–542
Cowley AW Jr, Switzer SJ, Guinn MM (1980) Evidence and quantification of the vasopressin arterial pressure control system in the dog. Circ Res 46:58–67
Dampney RAL (1981) Brain stem mechanisms in the control of arterial pressure. Clin Exp Hyper 3:379–391
Dampney RAL, Goodchild AK, Robertson LG, Montgomery W (1982) Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res 249:223–235
Dampney RAL, Moon EA (1980) Role of ventrolateral medulla in vasomotor response to cerebral ischemia. Am J Physiol 239:H349-H358
Errington ML, Dashwood MR (1979) Projections to the ventral surface of the cat brainstem demonstrated by horseradish peroxidase. Neurosci Letters 12:153–158
Feldberg W (1976) The ventral surface of the brain stem: a scarcely explored region of pharmacological sensitivity. Neuroscience 1:427–441
Feldberg W, Rocha E, Silva M Jr (1978) Vasopressin release produced in anaesthetized cats by antagonists of γ-aminobutyric acid and glycine. Br J Pharmacol 62:99–106
Feldberg W, Rocha E, Silva M Jr (1981) Inhibition of vasopressin release to carotid occlusion by γ-aminobutyric acid and glycine. Br J Pharmacol 72:17–24
Fifkova E, Marsala J (1967) Stereotaxic atlases for the cat, rabbit and rat. In: Bures J, Petran M, Zachar J (eds) Electrophysiological methods in biological research. Academia, Prague, pp 653–695
Goodchild AK, Dampney RAL, Bandler R (1982) A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J Neurosci Methods 6:351–363
Guertzenstein PG, Hilton SM, Marshall JM, Timms RJ (1978) Experiments on the origin of vasomotor tone. J Physiol 275:78P-79P
Houck PC, Fiksen-Olsen MJ, Britton SL, Romero JC (1983) Role of angiotensin and vasopressin on blood pressure of ganglionic blocked dogs. Am J Physiol 244:H115-H120
Kleinman LI, Radford EP Jr (1964) Ventilation standards for small mammals. J Appl Physiol 19:360–362
Kruszynski M, Lammek B, Manning M (1980) (1-(β-mercapto-β,β-cyclopentamethylenepropionic acid), 2-(O-methyl)tyrosine)arginine-vasopressin and (1-(β-mercapto-β,β-cyclopentamethylenepropionic acid)arginine-vasopressin, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem 23:364–368
Kubo T, Misu Y (1981a) Pharmacological characterisation of the alpha-adrenoceptors responsible for a decrease of blood pressure in the nucleus tractus solitarii of the rat. Naunyn-Schmiedeberg's Arch Pharmacol 317:120–125
Kubo T, Misu Y (1981b) Cardiovascular response to microinjection of physiostigmine and choline into the dorsal medulary site of the rat. Neuropharmacology 20:1091–1095
Kubo T, Misu Y (1984) Vasopressin-induced pressor responses following microinjection of kainic acid into the nucleus tractus solitarii of the rat. Jpn J Pharmacol 36:118–120
Loewi AP, Burton H (1978) Nuclei of the solitary tract: efferent projections to the lower brain stem and spinal cord of the cat. J Comp Neurol 181:421–450
McKellar S, Loewy AD (1982) Efferent projections of the A1 catecholamine cell group in the rat: an autoradiographic study. Brain Res 241:11–29
Nakai M, Iadecola C, Reis DJ (1982a) Global cerebral vasodilation by stimulation of rat fastigial cerebellar nucleus. Am J Physiol 243:H226-H235
Nakai M, Yamane Y, Umeda Y, Ogino K (1982b) Vasopressin-induced pressor response elicited by electrical stimulation of solitary nucleus and dorsal motor nucleus of vagus of rat. Brain Res 251:164–168
Reis DJ, Ross CA, Ruggiero DA, Granata AR, Joh TH (1984) Role of adrenaline neurons of ventrolateral medulla (the C1 group) in the tonic and phasic control of arterial pressure. Clin Exp Hyper A 6:221–241
Ricardo JA, Koh ET (1978) Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala and other forebrain structures in the rat. Brain Res 153:1–26
Sawchenko PE, Swanson LW (1981) Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science 214:685–687
Share L (1968) Control of plasma ADH titer in hemorrhage: role of atrial and arterial receptors. Am J Physiol 215:1384–1389
Sharpless SK, Rothballer AB (1961) Humoral factors released from intracranial sources during stimulation of reticular formation. Am J Physiol 200:909–915
Shinozaki H, Konishi S (1970) Actions of several anthelminthics and insecticides on rat cortical neurons. Brain Res 24:368–371
Thomas MR, Ulrichsen RF, Calaresu FR (1977) Function of lateral reticular nucleus in central cardiovascular regulation in the cat. Am J Physiol 232:H157-H166
Waeber B, Nussberger J, Brunner HR (1983) Blood pressure dependency on vasopressin and angiotensin II in prazosin-treated conscious normotensive rats. J Pharmacol Exp Ther 225:442–446
Wang BC, Sundet WD, Hakumaki MOK, Goetz KL (1983) Vasopressin and renin responses to hemorrhage in conscious, cardiac-denervated dogs. Am J Physiol 245:H399-H405
Weinstein H, Berne RM, Sachs H (1960) Vasopressin in blood: effect of hemorrhage. Endocrinology 66:712–718
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kubo, T., Amano, H. & Misu, Y. Caudal ventrolateral medulla. Naunyn-Schmiedeberg's Arch. Pharmacol. 328, 368–372 (1985). https://doi.org/10.1007/BF00692902
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00692902