Summary
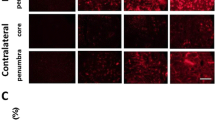
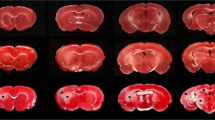
The effects of the calcium entry blocker emopamil on physiological variables, local cerebral blood flow (LCBF) and on hippocampal cell damage were evaluated after 10 min of forebrain ischemia in the rat. LCBF was determined with the 14C-iodoantipyrine technique after 2, 10, and 60 min of postischemic recirculation. Histological evaluation was performed 7 days after ischemia in cortical and hippocampal tissue by determination of the percentage of necrotic neurons. Preischemic application of emopamil [4 mg/kg racemate or 2 mg/kg (S)-emopamil; i.v.] caused increases in LCBF in cortical areas but did not alter blood flow in the hippocampus at 2 min of recirculation. After 10 and 30 min of flow resumption no differences in LCBF between drug-treated and control animals were observed. In the histological series (S)-emopamil was applied at doses of 2, 4 or 6 mg/kg before the induction of ischemia. After 7 days of postischemic recovery, neuronal damage was significantly reduced by the calcium antagonist in hippocampal CA 1 sector at all doses tested, the most prominent effects being observed with the lowest dose. At this dose cell loss in the Ca3 sector was also reduced. In cortical tissue the number of necrotic cells remained unchanged by emopamil treatment. It is concluded that the calcium antagonist emopamil can reduce ischemia-induced neuronal cell damage. The compound improves circulation in cortical tissue only during early recovery but not at later phases of reflow, i.e. the period of delayed hypoperfusion. These increases in blood flow are not of crucial importance for ultimate neuronal death in this area. The ameliorative action of emopamil on the survival of hippocampal neurons is not associated with blood flow changes and therefore seems to reflect a direct effect on cerebral parenchyma.
Similar content being viewed by others
References
Auer RN, Olsson Y, Siesjö BK (1984) Hypoglycemic brain injury in the rat: correlation of density of brain damage with the EEG isoelectric time. Diabetes 33:1090–1098
Beck T, Nuglisch J, Sauer D, Bielenberg GW, Mennel HD, Roßberg C, Krieglstein J (1989) Effects of flunarizine on postischemic blood flow, energy metabolism and neuronal damage in the rat brain. Eur J Pharmacol (in press)
Bielenberg GW, Haubruck H, Krieglstein J (1987a) Effects of calcium entry blocker emopamil on postischemic energy metabolism of the isolated perfused rat brain. J Cereb Blood Flow Metabol 7:489–496
Bielenberg GW, Beck T, Sauer D, Burniol M, Krieglstein J (1987b) Effects of cerebroprotective agents on cerebral blood flow and on postischemic energy metabolism in the rat brain. J Cereb Blood Flow Metabol 7:480–488
Deshpande JK, Wieloch T (1985) Amelioration of ischemic brain damage following postischemic treatment with flunarizine. Neurol Res 7:27–29
Deshpande JK, Wieloch T (1986) Flunarizine, a calcium entry blocker, ameliorates ischemic brain damage in the rat. Anesthesiology 64:215–224
Dienel GA (1984) Regional accumulation of calcium in the postischemic rat brain. J Neurochem 43:913–925
Farber JL (1981) The role of calcium in cell death. Life Sci 29:1289–1295
Gelmers HJ (1984) Effect of nimodipine on the clinical course of patients with acute ischemic stroke. Acta Neurol Scand 64:232–239
Grotta J, Spydell J, Pettigrew C, Ostrow P, Hunter D (1986) The effect of nicardipine on neuronal function following ischemia. Stroke 17:213–219
Hossmann KA, Paschen W, Csiba L (1983) Relationship between calcium accumulation and recovery of cat brain after prolonged cerebral ischemia. J Cereb Blood Flow Metabol 3:346–353
Hossmann KA, Grosse Ophoff B, Schmidt-Kastner R, Oschlies U (1985) Mitochondrial calcium sequestration in cortical and hippocampal neurons after prolonged ischemia of the cat brain. Acta Neuropathol (Berl) 68:230–238
Krieglstein J, Sauer D, Nuglisch J, Karkoutly C, Beck T, Bielenberg GW, Roßberg C, Mennel HD (1989) Protective effects of calcium antagonists against brain damage caused by ischemia. In: Hartmann A, Kuschinsky W (eds) Cerebral ischemia and calcium. (in press)
Kumar K, Krause G, Koestner A, Hoehner T, White B (1987) Effects of flunarizine on global brain ischemia in the dog: a quantitative morphologic assessment. Exp Neurol 97:115–127
Mennel HD, Sauer D, Roβberg C, Bielenberg GW, Krieglstein J (1989) Morphology of tissue damage due to experimental cerebral ischemia in rats. Exp Pathol (in press)
Mohamed AA, McCulloch J, Mendelew AD, Teasdale GM, Harper AM (1984) Effect of the calcium antagonist nimodipine on local cerebral blood flow: relationship to arterial blood pressure. J Cereb Blood Flow Metabol 4:206–211
Mohamed AA, Gotoh O, Graham DJ, Osborne KA, McCulloch J, Mendelow AD, Teasdale GM, Harper AM (1985) Effect of pretreatment with the calcium antagonist, nimodipine, on local cerebral blood flow and histopathology after middle cerebral artery occlusion. Ann Neurol 18:705–711
Myers RE (1979) Lactic acid accumulation as cause of brain edema and cerebral necrosis resulting from oxygen deprivation. In: Korobkin R, Giulleminault G (eds) Advances in perinatal neurology. Spectrum Publishers, New York, pp 85–114
Nedergaard M, Diemer NH (1987) Focal ischemia of the rat brain, with special reference to the influence of plasma glucose concentration. Acta Neuropathol (Berl) 73:131–137
Newberg LA, Steen PA, Milde JH, Michenfelder JD (1984) Failure of flunarizine to improve cerebral blood flow or neurologic recovery in a canine model of complete cerebral ischemia. Stroke 15:666–671
Paschen W, Hossmann KA, van den Kerckhoff W (1983) Regional assessment of energy-producing metabolism following prolonged complete ischemia of cat brain. J Cereb Blood Flow Metabol 3:321–329
Pulsinelli WA, Waldman S, Rawlinson D, Plum F (1982) Moderate hyperglycemia augments ischaemic brain damage: a neuropathologic study in the rat. Neurology (NY) 32:1234–1246
Reivich M, Jehle J, Sokoloff L, Kety SS (1969) Measurement of regional cerebral blood flow with antipyrine-14C in awake cats. J Appl Physiol 27:296–300
Sakamoto N, Kogure K, Kato H, Ohtomo H (1986) Disturbed Ca2+ homeostasis in the gerbil hippocampus following brief transient ischemia. Brain Res 364:372–376
Sakurada O, Kennedy C, Jehle J, Brown JD, Carbin GL, Sokoloff L (1978) Measurement of local cerebral blood flow with iodo 14C antipyrine. Am J Physiol 234:H59-H66
Schanne FAX, Kane AB, Young EE, Farber JL (1979) Calcium dependence of toxic cell death: a final common pathway. Science 206:700–702
Siemkowicz E, Hansen A (1978) Clinical restitution following cerebral ischemia in hypo-, normo-, and hyperglycemic rats. Acta Neurol Scand 58:1–8
Siesjö BK (1981) Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metabol 1:155–185
Siesjö BK, Wieloch T (1985) Cerebral metabolism in ischaemic: neurochemical basis for therapy. Br J Anesth 57:47–62
Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjö BK (1984) Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand 69:385–401
Steen PA, Gisvold SE, Milde JH, Newberg LA, Scheithauer BW, Lanier WL, Michenfelder JD (1985) Nimodipine improves outcome when given after complete cerebral ischemia in primates. Anesthesiology 62:406–414
Van Reempts J, Haseldonckx M, van Deuren B, Wouters L, Borgers M (1986) Structural damage of the ischemic brain: involvement of calcium and effects of postischemic treatment with calcium entry blockers. Drug Development Res 8:387–395
Weber J, Bielenberg GW, Krieglstein J (1988) effects of phenylalkylamine calcium entry blockers on postischemic energy metabolism in the isolated perfused rat brain: Stereoselective action of emopamil. Pharmacology 37:38–49
Wiernsperger N, Gygax P, Hofmann A (1984) calcium antagonist PY 108–068: Demonstration of its efficacy in various types of experimental ischemia. Stroke 15:679–685
Author information
Authors and Affiliations
Additional information
Send offprint requests to G. W. Bielenberg at the above address
Rights and permissions
About this article
Cite this article
Bielenberg, G.W., Sauer, D., Nughsch, J. et al. Effects of emopamil on postischemic blood flow and neuronal damage in rat brain. Naunyn-Schmiedeberg's Arch Pharmacol 339, 230–235 (1989). https://doi.org/10.1007/BF00165148
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00165148