Summary
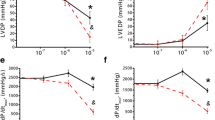
Experiments were carried out to characterize the adenosine-induced negative inotropic effect in relation to the extent of β-adrenoceptor activation in the isolated dog left ventricular myocardium. Adenosine and R-N6-phenylisopropyladenosine inhibited the positive inotropic effect of isoprenaline (10−7 mol/1 and lower) about 20% of its maximal response, which was antagonized by an A1 adenosine receptor antagonist 1,3-dipropyl-8-cyclopentylxanthine in a concentration-dependent manner. The negative inotropic effect of adenosine disappeared and that of R-N6-phenylisopro-pyl-adenosine decreased when the isoprenaline concentration was elevated to the level higher than 10−7 mol/1. Adenosine deaminase (1.5 U/ml) that abolished the negative inotropic effect of adenosine enhanced the effect of R-N6-phenylisopropyladenosine, indicating that endogenous adenosine released by high isoprenaline concentration (10−6 mol/1) modulates the interaction. The maximal response to adenosine and R-N6-phenylisopro-pyladenosine determined in the presence of 10−7 mol/1 isoprenaline was 50% of that of carbachol which elicited the maximal inhibition even in the presence of 10−6 mol/1 isoprenaline. The negative inotropic effects of R-N6-phenylisopropyladenosine and carbachol were additive to the maximal response equivalent to that of carbachol. The difference in the efficiency between the adenosine and muscarinic receptor agonists may be partly ascribed to the difference in densities of the respective receptors in the dog ventricular myocardium. The negative inotropic effect of R-N6-phenylisopropyladenosine in the presence of isoprenaline was associated with decrease in cyclic AMP levels elevated previously by isoprenaline. The elevation of cyclic AMP levels caused by isoprenaline (3 × 10−7 mol/1) was abolished by R-N6-phenylisopro-pyladenosine (10−4 mol/1), while the contractile response was reduced only by 30% with R-N6-phenylisopro-pyladenosine. In the absence of β-adrenoceptor stimulation R-N6-phenylisopropyladenosine elicited a negative inotropic effect without changes in cyclic AMP levels, but this effect was less than 10% of the basal force of contraction. It is concluded that in the dog ventricular myocardium adenosine receptors play a role for the inhibitory regulation of contractility, which is influenced markedly by the pre-existing level of β-adrenoceptor activation.
Similar content being viewed by others
References
Baumann G, Schrader J, Gerlach E (1981) Inhibitory action of adenosine on histamine- and dopamine-stimulated cardiac contractility and adenylate cyclase in guinea pigs. Circ Res 48:259–266
Böhm M, Bruckner R, Hackbarth I, Haubitz B, Linhart R, Meyer W, Schmidt B, Schmitz W, Scholz H (1984) Adenosine inhibition of catecholamine-induced increase in force of contraction in guinea-pig atrial and ventricular heart preparations. Evidence against a cyclic AMP- and cyclic GMP-dependent effect. J Pharmacol Exp Ther 230:483–492
Böhm M, Bruckner R, Neumann J, Nose M, Schmitz W, Scholz H (1988) Adenosine inhibits the positive inotropic effect of 3-isobutyl-l-methylxanthine in papillary muscles without effect on cyclic AMP or cyclic GMP. Br J Pharmacol 93:729–738
Brown AM, Yatani A, Codina J, Birnbaumer L (1989) In: Hondeghem L (ed) Molecular and cellular mechanisms of antiarrhythmic agents. Futura, New York, pp 73–80
Bruckner R, Fenner A, Meyer W, Nobis TM, Schmitz W, Scholz H (1985) Cardiac effects of adenosine and adenosine analogs in guinea-pig atrial and ventricular preparations: evidence against a role of cyclic AMP and cyclic GMP. J Pharmacol Exp Ther 234:766–774
Chiba S, Himori N (1975) Different isotropic responses to adenosine on the atrial and ventricular muscle of the dog heart. Jpn J Pharmacol 25:489–491
Endoh M (1979) Correlation of cyclic AMP and cyclic GMP levels with changes in contractile force of dog ventricular myocardium during cholinergic antagonism of positive inotropic actions of histamine, glucagon, theophylline and papaverine. Jpn J Pharmacol 29:855–864
Endoh M (1980) The time course of changes in cyclic nucleotide levels during cholinergic inhibition of positive inotropic actions of isoprenaline and theophylline in the isolated canine ventricular myocardium. Naunyn-Schmiedeberg's Arch Pharmacol 312:175–182
Endoh M, Blinks JR (1984) Effects of endogenous neurotransmitters on calcium transients in mammalian atrial muscle. In: Fleming WW, Langer SZ, Graefe KH, Weiner N (eds) Neuronal and extraneuronal events in autonomic pharmacology. Raven Press, New York, pp 221–230
Endoh M, Honma M (1979) Effects of papaverine and its interaction with isoprenaline and carbachol on the contractile force and cyclic nucleotide levels of canine ventricular myocardium. Naunyn-Schmiedeberg's Arch Pharmacol 306:241–248
Endoh M, Maruyama M, Taira N (1983a) Modification by isleta-ctivating protein of direct and indirect inhibitory actions of adenosine on rat atrial contraction in relation to cyclic nucleotide metabolism. J Cardiovasc Pharmacol 5:131–142
Endoh M, Maruyama M, Taira N (1983b) Adenosine-induced changes in rate of beating and cyclic nucleotide levels in rat atria: modification by islet-activating protein. In: Daly JW, Kuroda Y, Phillis JW, Shimizu H, Ui M (eds) Physiology and pharmacology of adenosine derivatives. Raven Press, New York, pp 127–141
Gilman AG (1984) G proteins and dual control of adenylate cyclase. Cell 36: 577–579
Hazeki O, Ui M (1981) Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem 256:2856–2862
Hiramoto T, Kushida H, Endoh M (1988) Further characterization of the myocardial a-adrenoceptors mediating positive inotropic effects in the rabbit myocardium. Eur J Pharmacol 152:301–310
Huang M, Drummond GI (1978) Interaction between adenosine and catecholamines on cyclic AMP accumulation in guinea pig ventricular myocardium. Biochem Pharmacol 27:187–191
Imai S, Riley AL, Berne RM (1964) Effect of ischemia on adenine nucleotides in cardiac and skeletal muscle. Circ Res 15:443–450
Jakobs KH, Aktories K, Schultz G (1984) Mechanism of pertussis toxin action on the adenylate cyclase system: inhibition of the turn-on reaction of the inhibitory regulatory site. Eur J Biochem 140:177–181
Kemmer M, Jakob H, Nawrath H (1989) Pronounced cholinergic but only moderate purinergic effects in isolated atrial and ventricular heart muscle from cats. Br J Pharmacol 97:1191–1198
Kitakaze M, Hori M, Sato H, Iwakura K, Gotoh K, Inoue M, Kitabatake A, Kamada T (1991) Beneficial effects of α1-adrenoceptor activity on myocardial stunnung in dogs. Circ Res 68:1322–1339
Kurachi Y, Nakajima T, Sugimoto T (1986) On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflügers Arch 407:264–274
Linden J, Hollen CE, Patel A (1985) The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium. Correlation with cyclic adenosine monophosphate and receptor densities. Circ Res 56:728–735
Londos C, Cooper DMF, Wolff J (1980) Subclasses of external adenosine receptors. Proc Natl Acad Sci USA 77:2551–2554
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Neumann J, Schmitz W, Scholz H, Stein B (1989) Effects of adenosine analogues on contractile response and cAMP content in guinea-pig isolated ventricular myocytes. Naunyn-Schmiedeberg's Arch Pharmacol 340:689–695
Pfaffinger PJ, Martin JM, Hunter DD, Nathanson NM, Hille B (1985) GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature 317: 536–538
Schipke J, Heusch G, Thämer V (1987) Evidence against the adenosine-catecholamine antagonism in the canine heart in situ. Arzneim-Forsch/Drug Res 37:1345–1347
Schrader J, Baumann G, Gerlach E (1977) Adenosine as inhibitor of myocardial effects of catecholamines. Pflügers Arch 372:29- 35
Schrader J, Baumann G, Gerlach E (1981) Antiadrenergic action of adenosine in the heart: possible physiological significance. In: Delius W, Gerlach E, Grobecker H, Kiibler W (eds) Catecholamines and the heart. Recent advances in experimental and clinical research. Springer, Berlin Heidelberg New York, pp 142–153
Seitelberger R, Schütz W, Schlappack O, Raberger G (1984) Evidence against the adenosine-catecholamine antagonism under in vivo conditions. Naunyn-Schmiedeberg's Arch Pharmacol 325:234–239
Tawfik-Schlieper H, Klotz KN, Kreye VAW, Schwabe U (1989) Characterization of the K+-channel-coupled adenosine receptor in guinea pig atria. Naunyn-Schmiedeberg's Arch Pharmacol 340: 684–688
Ui M, Katada T, Murayama T, Kurose H, Yajima M, Tamura M, Nakamura T, Nogimori K (1984) Islet-activating protein, pertussis toxin: a specific uncoupler of receptor-mediated inhibition of adenylate cyclase. Adv Cyclic Nucleotide Protein Phosphorylation Res 17:145–151
Urthaler F, Woods WT, James TN, Walker AA (1981) Effects of adenosine on mechanical performance and electrical activity in the canine heart. J Pharmacol Exp Ther 216:254–260
Von der Leyen H, Schmitz W, Scholz H, Scholz J, Lohse MJ, Schwabe U (1989) Effects of 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), a highly selective adenosine receptor antagonist, on force of contraction in guinea-pig atrial and ventricular cardiac preparations. Naunyn-Schmiedeberg's Arch Pharmacol 340:204–209
Watanabe AM, Besch HR Jr (1975) Interaction between cyclic adenosine monophosphate and cyclic guanosine monophosphate in guinea pig ventricular myocardium. Circ Res 37: 309–317
Yatani A, Imoto Y, Codina J, Hamilton SL, Brown AM, Birnbaumer L (1988) The stimulatory G protein of adenylyl cyclase, G, also stimulates dihydropyridine-sensitive Ca2+ channels. Evidence for direct regulation independent of phosphorylation by cAMP-dependent protein kinase or stimulation by a dihydropyridine agonist. J Biol Chem 263:9887–9895
Author information
Authors and Affiliations
Additional information
Send offprint requests to M. Endoh at the above address
Rights and permissions
About this article
Cite this article
Endoh, M., Kushida, H., Norota, I. et al. Pharmacological characteristics of adenosine-induced inhibition of dog ventricular contractility: dependence on the pre-existing level of β-adrenoceptor activation. Naunyn-Schmiedeberg's Arch Pharmacol 344, 70–78 (1991). https://doi.org/10.1007/BF00167384
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00167384