Abstract
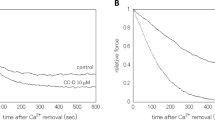
Maximally contracted detergent skinned coronary smooth muscle fibres are relaxed by lowering the concentration of free Ca2+. The extent and rate of relaxation depends on the concentration of free Ca2+ and calmodulin (CaM) suggesting that it is the Ca2+. CaM complex which is responsible for maintaining tension. At a fixed concentration of Ca2+ and CaM further relaxation can be achieved by addition of the catalytic subunit of the cAMP-dependent protein kinase (cAMP-kinase). The extent as well as the relaxation rate depend on the concentration of cAMP-kinase (0.01–0.5 μM) and both are antagonized by high concentrations of Ca2+ and CaM. The Ca2+-requirement for obtaining half maximal concentration is shifted from 1.1 μM to 6.3 μM Ca2+ in the presence of 0.5 μM cAMP-kinase. These data indicate that the response of the contractile apparatus to a change in the free [Ca2+] can be modulated by cAMP-kinase at the level of the contractile proteins. It is further suggested that the tone of coronary smooth muscle is determined by the relative and not by the absolute concentrations of Ca2+, CaM and cAMP-kinase.
Similar content being viewed by others
References
Adelstein RS, Hathaway DR (1979) Role of calcium and cyclic adenosine 3′,5′-monophosphate in regulating smooth muscle contraction. Am J Cardiol 44:783–787
Chatterjee M, Murphy RA (1983) Caleium-dependent stress maintenance without myosin phosphorylation in smooth muscle. Science 221:464–466
Conti MA, Adelstein RS (1981) The relationship between calmodulin binding and phosphorylation of smooth muscle kinase by the catalytic subunit of 3′,5′-cAMP-dependent protein kinase. J Biol Chem 256:3178–3181
Driska SP, Aksoy MO, Murphy RA (1981) Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am J Physiol 240:C222–C333
Gardner JP, DiSalvo J (1983) Temporal relations between isometric force and myosin light chain (MLC) phosphorylation in skinned porcine carotid arteries. Fed Proceedings 42:736
Gerthoffer WT, Murphy RA (1933) Ca2+, myosin phosphorylation and relaxation of arterial smooth muscle. Am J Physiol 245:C271–C277
Gerthoffer WT, Trevethick MA, Murphy RA (1984) Myosin phosphorylation and cyclic adenosine 3′,5′-monophosphate in relaxation of arterial smooth muscle by vasodilators. Circ Res 54:83–89
Hartshorne D, Mrwa U (1982) Regulation of smooth muscle actomyosin. Blood Vessels 19:1–18
Hofmann F, Bechtel P, Krebs FG (1977) Concentration of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem 252:1441–1447
Kerrick WGL, Hoar PE (1981) Inhibition of smooth muscle tension by cyclic AMP-dependent protein kinase. Nature (Lond) 292:253–255
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lanerolle P de, Stull JT (1980) Myosin phosphorylation during contraction and relaxation of tracheal smooth muscle. J Biol Chem 255:9993–10000
Lanerolle P de, Nishikawa M, Yost DA, Adelstein RS (1984) Increased phosphorylation of myosin light chain kinase after an increase in cyclic AMP in intact smooth muscle. Science 223:1415–1417
Marston SB (1982) The regulation of smooth muscle contractile proteins. Prog Biophys Mol Biol 41:1–41
Meisheri KD, Rüegg JC (1983) Dependence of cyclic-AMP-induced relaxation on Ca2+ and calmodulin in skinned smooth muscle of guinea pig taenia coli. Pflügers Arch 399:315–320
Miller JR, Silver PJ, Stull JT (1983) The role of myosin light chain kinase phosphorylation in β-adrenergic relaxation of tracheal smooth muscle. Mol Pharmacol 24:235–242
Moisescu DG, Thieleczek R (1978) Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol 275:241–263
Morgan JP, Morgan K (1984) Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol (Lond) 357:539–551
Müller E, Breemen C van (1979) Role of intracellular Ca2+ sequestration in β-adrenergic relaxation of a smooth muscle. Nature (Lond) 281:682–683
Nishikori K, Weisbrodt NW, Sherwood OD, Sanborn BM (1982) Relaxin alters rat uterine myosin light chain phosphorylation and related enzymatic activity. Endocrinology 111:1743–1745
O'Farrell PH (1975) High resolution two dimensional of proteins. J Biol Chem 250:4007–4021
Pfitzer G, Peterson JW, Rüegg JC (1982) Length dependence of calcium activated isometric force and immediate stiffness in living and glycerol extracted vascular smooth muscle. Pflügers Arch 394:174–181
Pfitzer G, Merkel L, Meisheri KD, Hofmann F, Rüegg JC (1984) The catalytic subunit of cAMP-dependent protein kinase promotes the rate of relaxation in coronary smooth muscle as well as myosin light chain phosphorylation in a Ca2+-dependent fashion. J Muscle Res Cell Mot 5:234
Portzehl H, Caldwell PC, Rüegg JC (1964) The dependence of contraction and relaxation of muscle fibres from the crab maia squinado on the internal concentration of free calcium ions. Biochim Biophys Acta 79:581–591
Rüegg JC, Paul RJ (1982) Vascular smooth muscle: calmodulin and cyclic AMP-dependent protein kinase alter calcium sensitivity in porcine carotid skinned fibres. Circ Res 50:394–399
Rüegg JC, Sparrow MP, Mrwa U (1981) Cyclic AMP mediated relaxation of chemically skinned fibres of smooth muscle. Pflügers Arch 390:198–201
Rüegg JC, Pfitzer G, Zimmer M, Hofmann F (1984) The calmodulin fraction responsible for contraction in an intestinal smooth muscle. FEBS Lett 170:383–386
Saida K (1982) Intracellular Ca release in skinned smooth muscle. J. Gen Physiol 80:191–202
Sharma RK, Wang JH (1979) Preparation and assay of the Ca2+-dependent modulator protein. Adv Cycl Nucl Res 10:187–198
Silver PJ, Stull JT (1982) Regulation of myosin light chain and phosphorylase phosphorylation in tracheal smooth muscle. J Biol Chem 257:6145–6150
Silver PJ, Schmidt-Silver C, DiSalvo J (1982) β-adrenergic relaxation and cAMP kinase activation in coronary arterial smooth muscle. Am J Physiol 242:H177–H184
Sparrow MP, Mrwa U, Hofmann F, Rüegg JC (1981) Calmodulin is essential for smooth muscle contraction. FEBS Lett 125:141–145
Sparrow MP, Pfitzer G, Gagelmann M, Rüegg JC (1984) Effect of calmodulin, Ca2+, and cAMP protein kinase on skinned tracheal smooth muscle. Am J Physiol 246:C308–C314
Thomas MV (1982) Techniques in calcium research. Academic Press, New York, pp 40–45
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pfitzer, G., Rüegg, J.C., Zimmer, M. et al. Relaxation of skinned coronary arteries depends on the relative concentrations of Ca2+, calmodulin and active cAMP-dependent protein kinase. Pflugers Arch. 405, 70–76 (1985). https://doi.org/10.1007/BF00591100
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00591100