Abstract
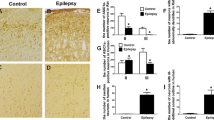
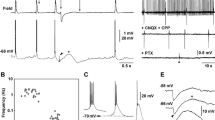
Lowering [Mg2+]o induces epileptiform bursting in hippocampus and entorhinal cortex (EC), presumably by activation of N-methyl-d-aspartate (NMDA) receptors. Since increasing [H+]o has been shown to reduce NMDA receptor activation, we hypothesized that this could contribute to anticonvulsant actions of acidic pH. To test this, we studied the effects of raising extracellular PCO2 (20.6%, pH = 6.7) or lowering extracellular pH (6.7 or 6.2) on low-Mg2+-induced epileptiform discharges. Lowering the pH to 6.7 by either means increased the interval between seizure-like events (SLEs), decreased the maximal amplitude of SLEs, and, if the site of seizure generation was at a distance from the recording site, acidification slowed the rate of seizure propagation. In contrast, the duration of SLEs was unaffected by acidic pH or high PCO2. Raising PCO2 or lowering pH to 6.7 also blocked early (8–10 min) but not late (> 20 min) phases of status-like discharges. All effects of the extracellular pH changes were fully reversible. Further lowering of extracellular pH to 6.2 completely and reversibly blocked both SLEs and status-like discharges. Our data show that the effects of high PCO2 and low pH on seizures in the EC in vitro may be dose-dependent and consistent with induction by proton blockade of NMDA receptors. Thus, blockade of NMDA currents by protons may be an important component of the anticonvulsant action of extracellular acidosis. The results also suggest that acidosis may be a desirable property for new antiepileptic treatments.
Similar content being viewed by others
References
Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA (1986) Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res 398:215–219
Aram JA, Lodge D (1987) Epileptiform activity induced by alkalosis in rat neocortical slices: block by antagonists of N-methyl-aspartate. Neurosci Lett 83:345–350
Balestrino M, Aitken PG, Somjen GG (1986) The effects of moderate changes of extracellular K+ and Ca2+ on synaptic and neuronal function in the CA1 region of the hippocampal slice. Brain Res 377:229–239
Balestrino M, Somjen GG (1988) Concentration of carbon dioxide, interstitial pH and synaptic transmission in hippocampal formation of the rat. J Physiol (Lond) 396:247–266
Caspers H, Speckmann EJ (1972) Cerebral pO2, pCO2 an pH: changes during convulsive activity and their significance for spontaneous arrest of seizures. Epilepsia 13:699–725
Chen JCT, Chesler M (1992a) Modulation of extracellular pH by glutamate and GABA in rat hippocampal slices. J Neurophysiol 67:29–36
Chen JCT, Chesler M (1992b) Extracellular alkaline shifts in rat hippocampal slice are mediated by NMDA and non-NMDA receptors. J Neurophysiol 68:342–344
Chen JCT, Chesler M (1992) pH transients evoked by excitatory synaptic transmission are increased by inhibition of extracellular carbonic anhydrase. Proc Natl Acad Sci USA 89:7786–7790
Choi DW (1988) Calcium-mediated neurotoxicity: relation to specific channel types and role in ischemic damage. Trends Neurosci 11:465–469
Church H, McLennan H (1989) Electrophysiological properties of rat CA1 pyramidal neurones in vitro modified by changes in extracellular bicarbonate. J Physiol (Lond) 415:85–108
Collingridge GL, Kehl SJ, McLennan H (1983) Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol (Lond) 334:33–46
Dean JC (1993) Valproate. In: Wyllie E (ed) The treatment of epilepsy: principles and practices. Lea and Febiger, Philadelphia, pp 915–922
Dreier JP, Heinemann U (1990) Late low magnesium-induced epileptiform activity in rat entorhinal cortex slices becomes insensitive to the anticonvulsant valproic acid. Neurosci Lett 119:68–70
Dreier JP, Heinemann U (1991) Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res 87:581–596
Esplin DW, Capek R, Esplin BA (1972) An intracellular study of the actions of carbon dioxide on the spinal monosynaptic pathway. Can J Physiol Pharmacol 51:424–436
Grantyn R, Lux HD (1988) Exclusion of NMDA and protein activated transient Na+ currents in rat tectal neurons. Neurosci Lett 89:198–203
Heinemann U, Arens J, Dreier JP, Stabel J, Zhang CL (1991) In vitro epileptiform activity: role of excitatory amino acids. Epilepsy Res 10:18–23
Herron CE, Williamson R, Collingridge GL (1985) A selective N-methyl-d-aspartate antagonist depresses epileptiform activity in rat hippocampal slices. Neurosci Lett 61:255–260
Heuser D, Astrup J, Lassen NA, Betz E (1975) Brain carbonic acid acidosis after acetazolamide. Acta Physiol Scand 93:385–390
Hille B (1968) Changes and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol 51:221–236
Jarolimek W, Misgeld U, Lux HD (1989) Activity dependent alkaline and acid transients in guinea pig hippocampal slices. Brain Res 505:225–232
Jones RSG (1989) Ictal epileptiform events induced by removal of extracellular magnesium in slices of entorhinal cortex are blocked by baclofen. Exp Neurol 104:155–161
Jones RSG, Heinemann U (1988) Synaptic and intrinsic responses of medial entorhinal cortical cells in normal and magnesium free medium in vitro. J Neurophysiol 59:1476–1496
Kaku DA, Giffard RG, Choi DW (1993) Neuroprotective effects of glutamate antagonists and extracellular acidity. Science 260:1516–1518
Kaplan PW, Lesser RP (1993) Focal cortical resection evaluation: noninvasive EEG. In: Wyllie E (ed) The treatments of epilepsy: principles and practice. Lea and Febiger, Philadelphia, pp 1014–1022
Köhr G, Heinemann U (1987) Ketamine and 2-aminophosphonovalerate in the low magnesium epilepsy in rat hippocampal slices. Neurosci Res Commun 1:17–21
Lothman EW, Bertram EH, Bekenstein JW, Perlin JB (1989) Selfsustaining limbic status epilepticus induced by “continuous” hippocampal electrical stimulation: electrographic and behavioral characteristics. Epilepsy Res 3:107–119
Louvel J, Heinemann U (1983) Changes in [Ca2+]o, [K+]o and neuronal activity during oenathotoxin-induced epilepsy in cat sensorimotor cortex. Electroencephalogr Clin Neurophysiol 56:457–466
MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321:519–522
Mayer ML, Westbrook GL, Guthrie PB (1984) Voltage dependent block by Mg2+ of NMDA responses in spinal cord neurons. Nature 309:261–263
Miller JJ, Baimbridge KG, Mody I (1986) Calcium regulation in kindling-induced epilepsy. In: Wada JA (ed) Kindling 3. Raven, New York, pp 301–304
Mody I, Lambert JDC, Heinemann U (1987) Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J Neurophysiol 57:869–888
Morrell F (1991) Kindling and synaptic plasticity. The legacy of Graham Goddard. Birkhäuser, Boston
Somjen GG (1984) Acidification of interstitial fluid in hippocampal formation caused by seizures and spreading depression. Brain Res 311:186–188
Somjen GG, Allen BW, Balestrino M, Aitken PG (1987) Pathophysiology of pH and Ca2+ in bloodstream and brain. Can J Physiol Pharmacol 65:1078–1085
Stanton PK, Jones RSG, Mody I, Heinemann U (1987) Epileptiform activity induced by lowering extracellular [Mg2+] in combined hippocampal-entorhinal cortex slices: modulation by receptors for norepinephrine and N-methyl-d-aspartate. Epilepsy Res 1:53–62
Taira T, Smirnov S, Voipio J, Kaila K (1993) Intrinsic proton modulation of excitatory transmission in rat hippocampal slices. Neuroreport 4:93–96
Tang CM, Dichter M, Morad M (1990) Modulation of the N-methyl-d-aspartate channel by extracellular H+. Proc Natl Acad Sci USA 87:6445–6449
Thomson AM, West DC, Lodge D (1985) An N-methyl-d-aspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature 313:479–481
Traynelis SF, Cull-Candy SG (1991) Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J Physiol (Lond) 433:727–763
Traynelis SF, Cull-Candy SG (1990) Proton inhibition of N-methyl-d-aspartate receptors in cerebellar neurons. Nature 345:347–350
Treiman DM, Walton NY, Kendrick C (1990) A progressive sequence of electroencephalographic changes during generalized convulsive status epilepticus. Epilepsy Res 5:49–60
Uthman BM, Wilder BJ (1993) Less commonly used antiepileptic drugs. In: Wyllie E (ed) The treatment of epilepsy: principles and practices. Lea and Febiger, Philadelphia, pp 959–973
Velíšek L, Mareš P (1992) Developmental aspects of the anticonvulsant action of MK-801. In: Kamenka J-M, Domino EF (eds) Multiple sigma and PCP receptor ligands: mechanisms for neuromodulation and neuroprotection? NPP Books, Ann Arbor, pp 779–795
Walther H, Lambert JDC, Jones RSG, Heinemann U, Hamon B (1986) Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium. Neurosci Lett 69:156–161
Walton NY, Treiman DM (1988a) Experimental secondarily generalized convulsive status epilepticus induced by dl-homocysteine thiolactone. Epilepsy Res 2:79–86
Walton NY, Treiman DM (1988b) Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol 101:267–275
Withrow CD (1972) Systemic carbon dioxide derangements. In: Purpura DP, Penry JK, Tower DB, Woodbury DM, Walter RD (eds) Experimental models of epilepsy a manual for laboratory worker. Raven, New York, pp 477–494
Wong RKS, Prince DA (1990) The lateral spread of ictal discharges in neocortical brain slices. Epilepsy Res 7:29–39
Woodbury DM, Karler R (1960) The role of carbon dioxide in the nervous system. Anesthesiology 21:686–703
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Velíšek, L., Dreier, J.P., Stanton, P.K. et al. Lowering of extracellular pH suppresses low-Mg2+-induces seizures in combined entorhinal cortex-hippocampal slices. Exp Brain Res 101, 44–52 (1994). https://doi.org/10.1007/BF00243215
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00243215