Abstract
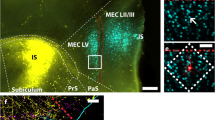
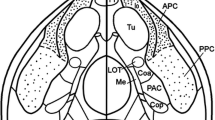
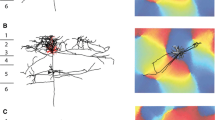
The relations between the inputs from the presubiculum and the parasubiculum and the cells in the entorhinal cortex that give rise to the perforant pathway have been studied in the rat at the light microscopical level. Projections from the presubiculum and the parasubiculum were labeled anterogradely, and, in the same animal, cells in the entorhinal cortex that project to the hippocampal formation were labeled by retrograde tracing and subsequent intracellular filling with Lucifer Yellow. The distribution and the number of appositions between the afferent fibers and hippocampal projection neurons in the various layers of the entorhinal cortex were analyzed. The results show that layers I–IV of the entorhinal cortex contain neurons that give rise to projections to the hippocampal formation. The morphology of these projection neurons is highly variable and afferents from the presubiculum and the parasubiculum do not show a preference for any specific morphological cell type. Both inputs preferentially innervate the dendrites of their target cells. However, presubicular and parasubicular projections differ with respect to the layer of entorhinal cortex they project to. The number of appositions of presubicular afferents with cells that have their cell bodies in layer III of the entorhinal cortex is 2–3 times higher than with cells in layer II. In contrast, afferents from the parasubiculum form at least 2–3 times as many synapses on the dendrites of cells located in layer II than on neurons that have their cell bodies in layer III. Cells in layers I and IV of the entorhinal cortex receive weak inputs from the presubiculum and parasubiculum. Not only is the presubiculum different from the parasubiculum with respect to the distribution of projections to the entorhinal cortex, they also differ in their afferent and efferent connections. In turn, cells in layer II of the entorhinal cortex differ in their electrophysiological characteristics from those in layer III. Moreover, layer II neurons give rise to the projections to the dentate gyrus and field CA3/CA2 of the hippocampus proper, and cells in layer III project to field CA1 and the subiculum. Therefore, we propose that the interactions of the entorhinal-hippocampal network with the presubiculum are different from those with the parasubiculum.
Similar content being viewed by others
References
Beall MJ, Lewis DA (1992) Heterogeneity of layer II neurons in human entorhinal cortex. J Comp Neurol 321:241–266
Buhl EH, Lübke J (1989) Intracellular Lucifer Yellow injection in fixed brain slices combined with retrograde tracing, light and electron microscopy. Neuroscience 20:3–16
Caballero-Bleda M, Witter MP (1993) Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex. An anterograde tracing study in the rat. J Comp Neurol 328:115–129
Carboni AA, Lavelle WG, Barnes CL, Cipolloni PB (1990) Neurons of the lateral entorhinal cortex of the rhesus monkey: a Golgi, histochemical, and immunocytochemical characterization. J Comp Neurol 291:583–608
Germroth P, Schwerdtfeger WK, Buhl EH (1989) Morphology of identified entorhinal neurons projecting to the hippocampus. A light microscopical study combining retrograde tracing and intracellular injection. Neuroscience 30:683–689
Germroth P, Schwerdtfeger WK, Buhl EH (1991) Ultrastructure and aspects of functional organization of pyramidal and nonpyramidal entorhinal projection neurons contributing to the perforant path. J Comp Neurol 305:215–231
Haberly LB, Price JL (1978) Association and commissural fiber systems of the olfactory cortex in the rat. I. Systems originating in the piriform cortex and adjacent areas. J Comp Neurol 178:711–740
Haug FMS (1976) Sulphide silver pattern and cytoarchitectonics of parahippocampal areas in the rat. Special reference to the subdivision of area entorhinalis (area 28) and its demarcation from the pyriform cortex. Adv Anat Embryol Cell Biol 52:1–73
Jones RSG (1993) Entorhinal-hippocampal connections: a speculative view of their function. Trends Neurosci 16:58–64
Jones RSG, Buhl EH (1993) Basket-like interneurones in layer II of the entorhinal cortex exhibit a powerful NMDA-mediated synaptic excitation. Neurosci Lett 149:35–39
Köhler C (1985a) A projection from the deep layers of the entorhinal area to the hippocampal formation in the rat brain. Neurosci Lett 56:13–19
Köhler C (1985b) Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. J Comp Neurol 236:504–522
Köhler C (1986) Intrinsic connections of the retrohippocampal region in the rat brain: II. The medial entorhinal area. J Comp Neurol 246:149–169
Köhler C (1988) Intrinsic connections of the retrohippocampal region in the rat brain: III. The lateral entorhinal area. J Comp Neurol 271:208–228
Kosel KC, Van Hoesen GW, West JR (1981) Olfactory bulb projections to the hippocampal area of the rat. J Comp Neurol 198:467–482
Lingenhöhl K, Finch DM (1991) Morphological characterization of rat entorhinal neurons in vivo: soma-dendritic structure and axonal domains. Exp Brain Res 84:57–74
Lopes da Silva FH, Witter MP, Boeijinga PH, Lohman AHM (1990) Anatomic organization and physiology of the limbic cortex. Physiol Rev 70:453–511
Lorente de Nó R (1933) Studies on the structure of the cerebral cortex. J Psychol Neurol 45:381–443
Martinez A, Soriano E, Fariñas I (1992) Axo-axonic chandelier cells in the entorhinal cortex and subicular complex of the rat: a Golgi electron microscopic study. Eur J Neurosci [Suppl] 5:70
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, Sydney
Schwartz SP, Coleman PD (1981) Neurons of origin of the perforant path. Exp Neurol 74:305–312
Steward O (1976) Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol 167:285–314
Steward O, Scoville SA (1976) Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol 169:347–370
Swanson LW, Cowan WM (1977) An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172:49–84
Tamamaki N, Nojyo Y (1993) Projection of the entorhinal layer II neurons in the rat as revealed by intracellular pressure-injection of neurobiotin. Hippocampus 3:471–480
Thompson SM, Robertson RT (1987) Organization of subcortical pathways for sensory projections to the limbic cortex I. Subcortical projections to the medial limbic cortex in the rat. J Comp Neurol 265:175–188
Van Groen T, Wyss JM (1990) The connections of presubiculum and parasubiculum in the rat. Brain Res 518:227–243
Vogt BA, Miller MW (1983) Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216:192–210
Witter MP, Jorritsma-Byham B (1992) Neurons in layer Va of the entorhinal cortex form part of a hippocampal cortical output system. Eur J Neurosci[Suppl] 5:69
Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM (1989) Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33:161–253
Wouterlood FG, Jorritsma-Byham B, Goede PH (1990) Combination of anterograde tracing with Phaseolus vulgaris-leucoagglutinin, retrograde fluorescent tracing and fixed-slide intracellular injection of Lucifer yellow. J Neurosci Meth 33:207–217
Wyss JM (1981) An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol 199:495–512
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caballero-Bleda, M., Witter, M.P. Projections from the presubiculum and the parasubiculum to morphologically characterized entorhinal-hippocampal projection neurons in the rat. Exp Brain Res 101, 93–108 (1994). https://doi.org/10.1007/BF00243220
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00243220