Abstract
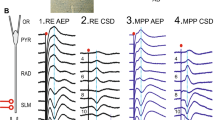
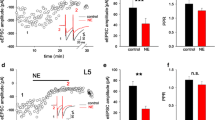
In vitro norepinephrine (NE) induces both short and long-term β-receptor-mediated potentiation of the perforant path-evoked population spike in the dentate gyrus. NE or locus coeruleus (LC) activation in vivo also produces a β-receptor dependent potentiation of population spike amplitude in anesthetized rat. Studies of behavioral state modulation of population spike amplitude in awake rats, and in rats depleted of NE, however, have led to the hypothesis that LC-NE activation should act to suppress or reduce population spike amplitude in the dentate gyrus of unanesthetized rat. Using glutamate activation of LC in awake unrestrained rats (n=12), the present study provides evidence that LC activation in the awake rat does not reduce, but potentiates, population spike amplitude. The potentiation effect was long-lasting (>25min) in 50% of the experiments. In addition glutamate ejections in the third lobe of cerebellar rostral vermis produced potentiation of population spike amplitude (n=3) and population excitatory postsynaptic potential slope. Ejections at sites outside the LC and rostral vermis were ineffective (n=5). Behavioral effects of glutamate ejection did not predict the occurrence of potentiation. These data support the hypothesis that phasic activation of LC cells is likely to induce short-term, and possibly long-term, potentiation of dentate gyrus throughput in alert animals.
Similar content being viewed by others
References
Assaf SY, Mason ST, Miller JJ (1979) Noradrenergic modulation of neuronal transmission between the entorhinal cortex and the dentate gyrus of the rat (abstract). J Physiol (Lond) 292:52P
Aston-Jones G, Bloom FE (1981a) Activity of norepinephrine-containing neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1:876–886
Aston-Jones G, Bloom FE (1981b) Norepinephrine-containing locus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1:887–900
Babstock DM, Harley CW (1992) Paragigantocellularis stimulation induces β-adrenergic hippocampal potentiation. Brain Res Bull 28:709–714
Babstock DM, Harley CW (1993) Lateral olfactory tract input to dentate gyrus is depressed by prior noradrenergic activation using nucleus paragigantocellularis stimulation. Brain Res 629:149–154
Cederbaum JM, Aghajanian GK (1976) Noradrenergic neurons of the locus coeruleus: inhibition by epinephrine and activation by the α-antagonist piperoxane. Brain Res 112:413–419
Chu N, Bloom FE (1973) Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science 179:908–909
Dahl D, Sarvey JM (1989) Norepinephrine induces pathway-specific long-lasting potentiation and depression in the hippocampal dentate gyrus. Proc Natl Acad Sci USA 86:4776–4780
Dahl D, Winson J (1985) Action of norepinephrine in the dentate gyrus. I. Stimulation of locus coeruleus. Exp Brain Res 59:491–496
Dahl D, Bailey WH, Winson J (1983) Effect of norepinephrine depletion of hippocampus on neuronal transmission from perforant path through dentate gyrus. J Neurophysiol 49:123–133
Green EJ, McNaughton BL, Barnes CA (1990) Exploration-dependent modulation of evoked responses in fascia dentata: dissociation of motor, EEG, and sensory factors and evidence for a synaptic efficacy change. J Neurosci 10:1455–1471
Harley CW, Evans S (1988) Locus-coeruleus-induced enhancement of the perforant-path evoked potential: effects of intradentate beta blockers. In: Woody CD, Alkon DL, McGaugh JL (eds) Cellular mechanisms of conditioning and behavioral plasticity. Plenum, New York, pp 415–423
Harley CW, Milway JS (1986) Glutamate ejection in the locus coeruleus enhances the perforant path-evoked population spike in the dentate gyrus. Exp Brain Res 63:143–150
Harley CW, Sara SJ (1992) Locus coeruleus bursts induced by glutamate trigger delayed perforant path spike amplitude potentiation in the dentate gyrus. Exp Brain Res 89:581–587
Harley C, Milway JS, Lacaille JC (1989) Locus coeruleus potentiation of dentate gyrus responses: evidence for two systems. Brain Res Bull 22:643–650
Heath RG, Harper JW (1974) Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol 45:268–287
Heath RG, Dempesy CW, Fontana CJ, Myers WA (1978) Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol Psychiatr 13:501–529
Kety SS (1970) The biogenic amines in the central nervous system: their possible roles in arousal, emotion and learning. In: Schmitt FO (ed) The neurosciences second study program. Rockefeller University Press, New York, pp 324–355
Lacaille J-C, Harley CW (1985) The action of norepinephrine in the dentate gyrus: beta-mediated facilitation of evoked potentials in vitro. Brain Res 358:210–220
Maiti A, Snider RS (1975) Cerebellar control of basal forebrain seizures: amygdala and hippocampus. Epilepsia 16:521–523
Neuman RS, Harley CW (1983) Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res 273:162–165
Sara SJ, Bergis O (1991) Enhancement of excitability and inhibitory processes in hippocampal dentate gyrus by noradrenaline: a pharmacological study in awake, freely moving rats. Neurosci Lett 126:1–5
Sara SJ, Vankov A, Herve A (1994) Characteristics of firing of locus coeruleus neurons in the rat during active exploration of the environment: a clue for the role of noradrenaline in memory processes? Brain Res Bull (in press)
Stanton PK, Sarvey JM (1985) Blockade of norepinephrine-induced long-lasting potentiation in the hippocampal dentate gyrus by an inhibitor of protein synthesis. Brain Res 361:276–283
Stanton PK, Sarvey JM (1987) Norepinephrine regulates longterm potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull 18:115–119
Washburn M, Moises HC (1989) Electrophysiological correlates of presynaptic alpha2-receptor-mediated inhibition of norepinephrine release at locus coeruleus synapses in dentate gyrus. J Neurosci 9:2131–2140
Weisz DL, Clark GA, Thompson RF (1984) Increased responsivity of dentate granule cells during nictititating membrane response conditioning in rabbit. Behav Brain Res 12:145–154
Winson J, Abzug C (1978) Neuronal transmission through hippocampal pathways dependent on behavior. J Neurophysiol 41:716–732
Winson J, Dahl D (1985) Action of norepinephrine in the dentate gyrus. II. Iontophoretic studies. Exp Brain Res 59:497–506
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klukowski, G., Harley, C.W. Locus coeruleus activation induces perforant path-evoked population spike potentiation in the dentate gyrus of awake rat. Exp Brain Res 102, 165–170 (1994). https://doi.org/10.1007/BF00232449
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00232449